BAP1 regulates IP3R3-mediated Ca2+ flux to mitochondria suppressing cell transformation
- PMID: 28614305
- PMCID: PMC5581194
- DOI: 10.1038/nature22798
BAP1 regulates IP3R3-mediated Ca2+ flux to mitochondria suppressing cell transformation
Abstract
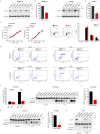
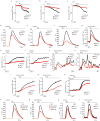
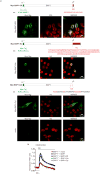
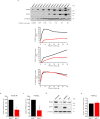
BRCA1-associated protein 1 (BAP1) is a potent tumour suppressor gene that modulates environmental carcinogenesis. All carriers of inherited heterozygous germline BAP1-inactivating mutations (BAP1+/-) developed one and often several BAP1-/- malignancies in their lifetime, mostly malignant mesothelioma, uveal melanoma, and so on. Moreover, BAP1-acquired biallelic mutations are frequent in human cancers. BAP1 tumour suppressor activity has been attributed to its nuclear localization, where it helps to maintain genome integrity. The possible activity of BAP1 in the cytoplasm is unknown. Cells with reduced levels of BAP1 exhibit chromosomal abnormalities and decreased DNA repair by homologous recombination, indicating that BAP1 dosage is critical. Cells with extensive DNA damage should die and not grow into malignancies. Here we discover that BAP1 localizes at the endoplasmic reticulum. Here, it binds, deubiquitylates, and stabilizes type 3 inositol-1,4,5-trisphosphate receptor (IP3R3), modulating calcium (Ca2+) release from the endoplasmic reticulum into the cytosol and mitochondria, promoting apoptosis. Reduced levels of BAP1 in BAP1+/- carriers cause reduction both of IP3R3 levels and of Ca2+ flux, preventing BAP1+/- cells that accumulate DNA damage from executing apoptosis. A higher fraction of cells exposed to either ionizing or ultraviolet radiation, or to asbestos, survive genotoxic stress, resulting in a higher rate of cellular transformation. We propose that the high incidence of cancers in BAP1+/- carriers results from the combined reduced nuclear and cytoplasmic activities of BAP1. Our data provide a mechanistic rationale for the powerful ability of BAP1 to regulate gene-environment interaction in human carcinogenesis.
Figures






Comment in
-
Tumor suppressive Ca2+ signaling is driven by IP3 receptor fitness.Cell Stress. 2017 Nov 1;1(2):73-78. doi: 10.15698/cst2017.11.109. Cell Stress. 2017. PMID: 31225437 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

