Human vaccination against Plasmodium vivax Duffy-binding protein induces strain-transcending antibodies
- PMID: 28614791
- PMCID: PMC5470884
- DOI: 10.1172/jci.insight.93683
Human vaccination against Plasmodium vivax Duffy-binding protein induces strain-transcending antibodies
Abstract
Background: Plasmodium vivax is the most widespread human malaria geographically; however, no effective vaccine exists. Red blood cell invasion by the P. vivax merozoite depends on an interaction between the Duffy antigen receptor for chemokines (DARC) and region II of the parasite's Duffy-binding protein (PvDBP_RII). Naturally acquired binding-inhibitory antibodies against this interaction associate with clinical immunity, but it is unknown whether these responses can be induced by human vaccination.
Methods: Safety and immunogenicity of replication-deficient chimpanzee adenovirus serotype 63 (ChAd63) and modified vaccinia virus Ankara (MVA) viral vectored vaccines targeting PvDBP_RII (Salvador I strain) were assessed in an open-label dose-escalation phase Ia study in 24 healthy UK adults. Vaccines were delivered by the intramuscular route in a ChAd63-MVA heterologous prime-boost regimen using an 8-week interval.
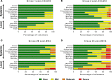
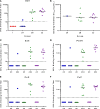
Results: Both vaccines were well tolerated and demonstrated a favorable safety profile in malaria-naive adults. PvDBP_RII-specific ex-vivo IFN-γ T cell, antibody-secreting cell, memory B cell, and serum IgG responses were observed after the MVA boost immunization. Vaccine-induced antibodies inhibited the binding of vaccine homologous and heterologous variants of recombinant PvDBP_RII to the DARC receptor, with median 50% binding-inhibition titers greater than 1:100.
Conclusion: We have demonstrated for the first time to our knowledge that strain-transcending antibodies can be induced against the PvDBP_RII antigen by vaccination in humans. These vaccine candidates warrant further clinical evaluation of efficacy against the blood-stage P. vivax parasite.
Trial registration: Clinicaltrials.gov NCT01816113.
Funding: Support was provided by the UK Medical Research Council, UK National Institute of Health Research Oxford Biomedical Research Centre, and the Wellcome Trust.
Keywords: Infectious disease; Vaccines.
Conflict of interest statement
Figures




References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

