Neuropeptide Y expression marks partially differentiated β cells in mice and humans
- PMID: 28614797
- PMCID: PMC5470892
- DOI: 10.1172/jci.insight.94005
Neuropeptide Y expression marks partially differentiated β cells in mice and humans
Abstract
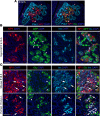
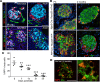
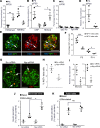
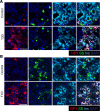
β Cells are formed in embryonic life by differentiation of endocrine progenitors and expand by replication during neonatal life, followed by transition into functional maturity. In this study, we addressed the potential contribution of neuropeptide Y (NPY) in pancreatic β cell development and maturation. We show that NPY expression is restricted from the progenitor populations during pancreatic development and marks functionally immature β cells in fetal and neonatal mice and humans. NPY expression is epigenetically downregulated in β cells upon maturation. Neonatal β cells that express NPY are more replicative, and knockdown of NPY expression in neonatal mouse islets reduces replication and enhances insulin secretion in response to high glucose. These data show that NPY expression likely promotes replication and contributes to impaired glucose responsiveness in neonatal β cells. We show that NPY expression reemerges in β cells in mice fed with high-fat diet as well as in diabetes in mice and humans, establishing a potential new mechanism to explain impaired β cell maturity in diabetes. Together, these studies highlight the contribution of NPY in the regulation of β cell differentiation and have potential applications for β cell supplementation for diabetes therapy.
Keywords: Development; Endocrinology.
Conflict of interest statement
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

