Robust memory responses against influenza vaccination in pemphigus patients previously treated with rituximab
- PMID: 28614800
- PMCID: PMC5470882
- DOI: 10.1172/jci.insight.93222
Robust memory responses against influenza vaccination in pemphigus patients previously treated with rituximab
Abstract
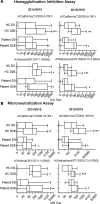
Rituximab is a therapeutic anti-CD20 monoclonal antibody widely used to treat B cell lymphoma and autoimmune diseases, such as rheumatic arthritis, systemic lupus erythematosus, and autoimmune blistering skin diseases (AIBD). While rituximab fully depletes peripheral blood B cells, it remains unclear whether some preexisting B cell memory to pathogens or vaccines may survive depletion, especially in lymphoid tissues, and if these memory B cells can undergo homeostatic expansion during recovery from depletion. The limited data available on vaccine efficacy in this setting have been derived from rituximab-treated patients receiving concomitant chemotherapy or other potent immunosuppressants. Here, we present an in-depth analysis of seasonal influenza vaccine responses in AIBD patients previously treated with rituximab, who generally did not receive additional therapeutic interventions. We found that, despite a lack of influenza-specific memory B cells in the blood, patients mount robust recall responses to vaccination, comparable to healthy controls, both at a cellular and a serological level. Repertoire analyses of plasmablast responses suggest that they likely derive from a diverse pool of tissue-resident memory cells, refractory to depletion. Overall, these data have important implications for establishing an effective vaccine schedule for AIBD patients and the clinical care of rituximab-treated patients in general and contribute to our basic understanding of maintenance of normal and pathogenic human B cell memory.
Keywords: Immunology; Vaccines.
Conflict of interest statement
Figures





References
-
- Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272(21):1661–1665. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

