Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma
- PMID: 28614802
- PMCID: PMC5470888
- DOI: 10.1172/jci.insight.93411
Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma
Abstract
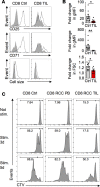
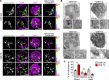
Cancer cells can inhibit effector T cells (Teff) through both immunomodulatory receptors and the impact of cancer metabolism on the tumor microenvironment. Indeed, Teff require high rates of glucose metabolism, and consumption of essential nutrients or generation of waste products by tumor cells may impede essential T cell metabolic pathways. Clear cell renal cell carcinoma (ccRCC) is characterized by loss of the tumor suppressor von Hippel-Lindau (VHL) and altered cancer cell metabolism. Here, we assessed how ccRCC influences the metabolism and activation of primary patient ccRCC tumor infiltrating lymphocytes (TIL). CD8 TIL were abundant in ccRCC, but they were phenotypically distinct and both functionally and metabolically impaired. ccRCC CD8 TIL were unable to efficiently uptake glucose or perform glycolysis and had small, fragmented mitochondria that were hyperpolarized and generated large amounts of ROS. Elevated ROS was associated with downregulated mitochondrial SOD2. CD8 T cells with hyperpolarized mitochondria were also visible in the blood of ccRCC patients. Importantly, provision of pyruvate to bypass glycolytic defects or scavengers to neutralize mitochondrial ROS could partially restore TIL activation. Thus, strategies to improve metabolic function of ccRCC CD8 TIL may promote the immune response to ccRCC.
Keywords: Immunology; Metabolism.
Conflict of interest statement
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

