The Significance of SDF-1α-CXCR4 Axis in in vivo Angiogenic Ability of Human Periodontal Ligament Stem Cells
- PMID: 28614918
- PMCID: PMC5523014
- DOI: 10.14348/molcells.2017.0004
The Significance of SDF-1α-CXCR4 Axis in in vivo Angiogenic Ability of Human Periodontal Ligament Stem Cells
Abstract
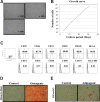
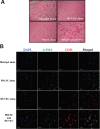
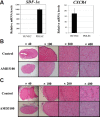
Periodontal ligament stem cells (PDLSCs) are multipotent stem cells derived from periodontium and have mesenchymal stem cell (MSC)-like characteristics. Recently, the perivascular region was recognized as the developmental origin of MSCs, which suggests the in vivo angiogenic potential of PDLSCs. In this study, we investigated whether PDLSCs could be a potential source of perivascular cells, which could contribute to in vivo angiogenesis. PDLSCs exhibited typical MSC-like characteristics such as the expression pattern of surface markers (CD29, CD44, CD73, and CD105) and differentiation potentials (osteogenic and adipogenic differentiation). Moreover, PDLSCs expressed perivascular cell markers such as NG2, αsmooth muscle actin, platelet-derived growth factor receptor β, and CD146. We conducted an in vivo Matrigel plug assay to confirm the in vivo angiogenic potential of PDLSCs. We could not observe significant vessel-like structures with PDLSCs alone or human umbilical vein endothelial cells (HU-VECs) alone at day 7 after injection. However, when PDLSCs and HUVECs were co-injected, there were vessel-like structures containing red blood cells in the lumens, which suggested that anastomosis occurred between newly formed vessels and host circulatory system. To block the SDF-1α and CXCR4 axis between PDLSCs and HUVECs, AMD3100, a CXCR4 antagonist, was added into the Matrigel plug. After day 3 and day 7 after injection, there were no significant vessel-like structures. In conclusion, we demonstrated the peri-vascular characteristics of PDLSCs and their contribution to in vivo angiogenesis, which might imply potential application of PDLSCs into the neovascularization of tissue engineering and vascular diseases.
Keywords: SDF-1α-CXCR4 axis; angiogenesis; mesenchymal stem cells; periodontal ligament stem cells; perivascular cells.
Figures


Similar articles
-
In Vivo Angiogenic Capacity of Stem Cells from Human Exfoliated Deciduous Teeth with Human Umbilical Vein Endothelial Cells.Mol Cells. 2016 Nov 30;39(11):790-796. doi: 10.14348/molcells.2016.0131. Epub 2016 Nov 18. Mol Cells. 2016. PMID: 27871176 Free PMC article.
-
Functional interleukin-7/interleukin-7Ralpha, and SDF-1alpha/CXCR4 are expressed by human periodontal ligament derived mesenchymal stem cells.J Cell Physiol. 2008 Mar;214(3):706-13. doi: 10.1002/jcp.21266. J Cell Physiol. 2008. PMID: 17894415
-
Angiogenic Capacity of Dental Pulp Stem Cell Regulated by SDF-1α-CXCR4 Axis.Stem Cells Int. 2017;2017:8085462. doi: 10.1155/2017/8085462. Epub 2017 May 15. Stem Cells Int. 2017. PMID: 28588623 Free PMC article.
-
Pericytes in the Periodontal Ligament.Adv Exp Med Biol. 2019;1122:169-186. doi: 10.1007/978-3-030-11093-2_10. Adv Exp Med Biol. 2019. PMID: 30937869 Review.
-
Comparison of Periodontal Ligament Stem Cells with Mesenchymal Stem Cells from Other Sources: A Scoping Systematic Review of In vitro and In vivo Studies.Curr Stem Cell Res Ther. 2024;19(4):497-522. doi: 10.2174/1574888X17666220429123319. Curr Stem Cell Res Ther. 2024. PMID: 36397622
Cited by
-
The Pro-Angiogenic Potential of Periodontal Ligament Stem Cells and Dental Pulp Stem Cells: A Comparative Analysis.Cells. 2025 Jun 8;14(12):864. doi: 10.3390/cells14120864. Cells. 2025. PMID: 40558491 Free PMC article.
-
Formation and Developmental Specification of the Odontogenic and Osteogenic Mesenchymes.Front Cell Dev Biol. 2020 Jul 17;8:640. doi: 10.3389/fcell.2020.00640. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850793 Free PMC article. Review.
-
LIPUS Promotes Endothelial Differentiation and Angiogenesis of Periodontal Ligament Stem Cells by Activating Piezo1.Int J Stem Cells. 2022 Nov 30;15(4):372-383. doi: 10.15283/ijsc22024. Epub 2022 Jun 30. Int J Stem Cells. 2022. PMID: 35769057 Free PMC article.
-
Transcriptome analysis reveals the mechanism of stromal cell-derived factor-1 and exendin-4 synergistically promoted periodontal ligament stem cells osteogenic differentiation.PeerJ. 2021 Aug 27;9:e12091. doi: 10.7717/peerj.12091. eCollection 2021. PeerJ. 2021. PMID: 34532163 Free PMC article.
-
Immunosuppressive Property of MSCs Mediated by Cell Surface Receptors.Front Immunol. 2020 Jul 28;11:1076. doi: 10.3389/fimmu.2020.01076. eCollection 2020. Front Immunol. 2020. PMID: 32849489 Free PMC article. Review.
References
-
- Armulik A., Abramsson A., Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. - PubMed
-
- Au P., Daheron L.M., Duda D.G., Cohen K.S., Tyrrell J.A., Lanning R.M., Fukumura D., Scadden D.T., Jain R.K. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111:1302–1305. - PMC - PubMed
-
- Chen F.M., Jin Y. Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Eng Part B Rev. 2010;16:219–255. - PubMed
-
- Corselli M., Chen C.W., Crisan M., Lazzari L., Peault B. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104–1109. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

