Interleukin-21 promotes thymopoiesis recovery following hematopoietic stem cell transplantation
- PMID: 28615039
- PMCID: PMC5471903
- DOI: 10.1186/s13045-017-0490-3
Interleukin-21 promotes thymopoiesis recovery following hematopoietic stem cell transplantation
Abstract
Background: Impaired T cell reconstitution remains a major deterrent in the field of bone marrow (BM) transplantation (BMT) due to pre-conditioning-induced damages inflicted to the thymi of recipient hosts. Given the previously reported thymo-stimulatory property of interleukin (IL)-21, we reasoned that its use post-BMT could have a profound effect on de novo T cell development.
Methods: To evaluate the effect of IL-21 on de novo T cell development in vivo, BM derived from RAG2p-GFP mice was transplanted into LP/J mice. Lymphocyte reconstitution was first assessed using a hematological analyzer and a flow cytometer on collected blood samples. Detailed flow cytometry analysis was then performed on the BM, thymus, and spleen of transplanted animals. Finally, the effect of human IL-21 on thymopoiesis was validated in humanized mice.
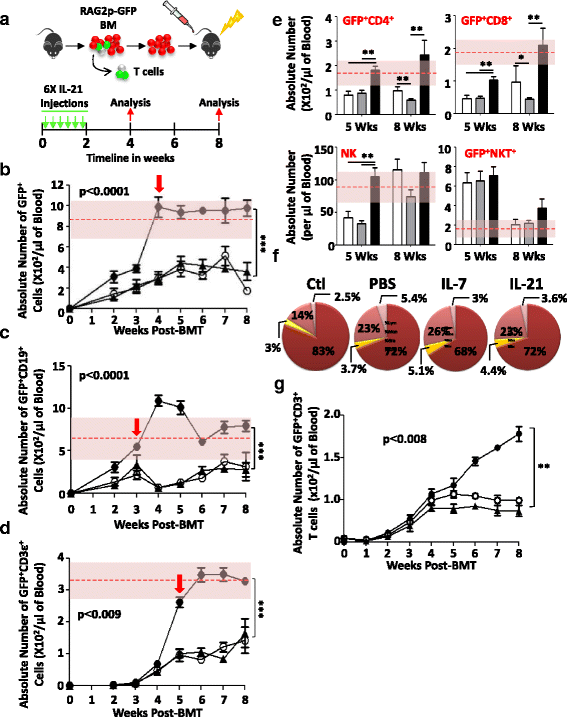
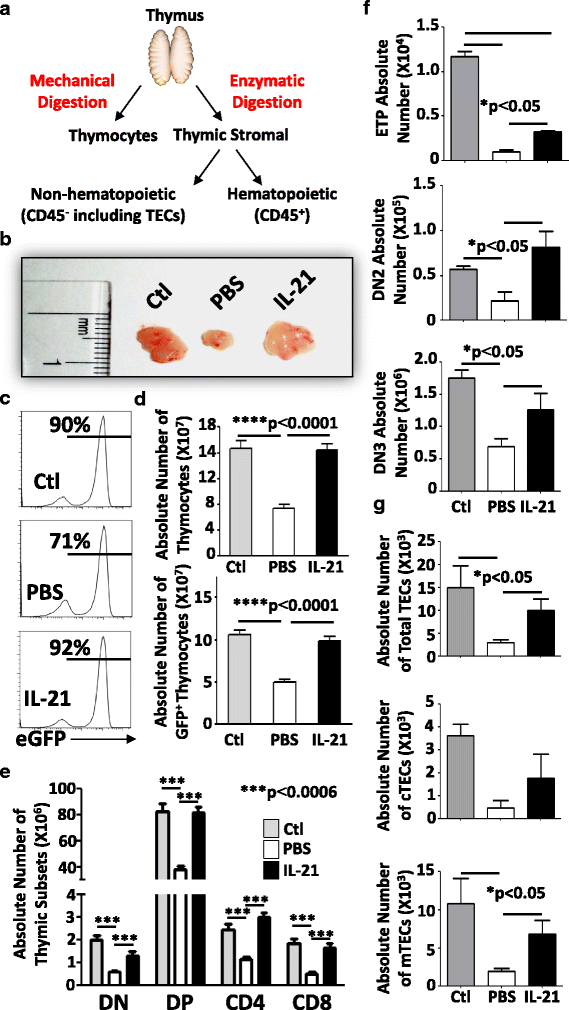
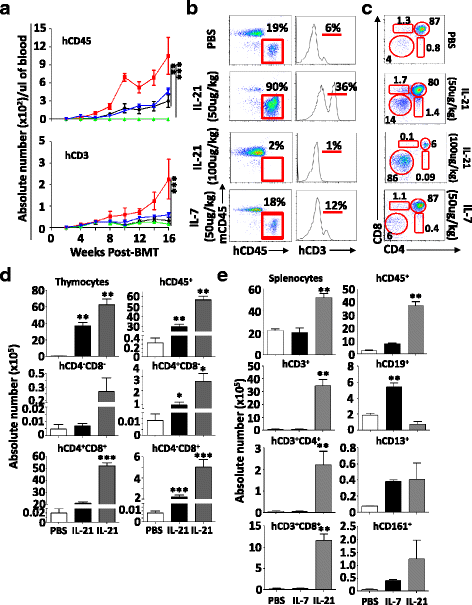
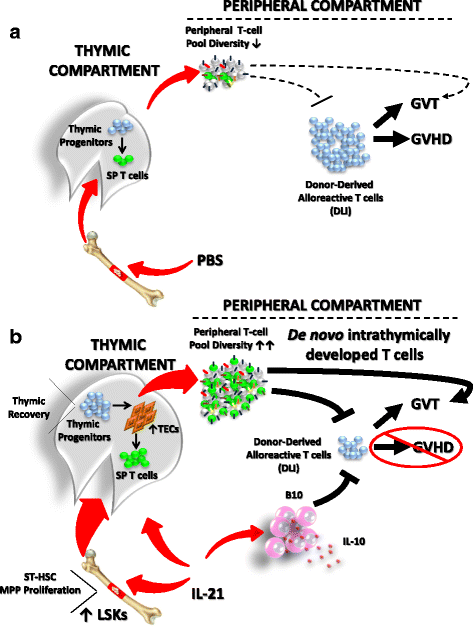
Results: Using a major histocompatibility complex (MHC)-matched allogeneic BMT model, we found that IL-21 administration improves immune reconstitution by triggering the proliferation of BM Lin-Sca1+c-kit+ (LSK) subsets. The pharmacological effect of IL-21 also culminates in the recovery of both hematopoietic (thymocytes) and non-hematopoietic (stromal) cells within the thymi of IL-21-treated recipient animals. Although T cells derived from all transplanted groups proliferate, secrete various cytokines, and express granzyme B similarly in response to T cell receptor (TCR) stimulation, full regeneration of peripheral naïve CD4+ and CD8+ T cells and normal TCRvβ distribution could only be detected in IL-21-treated recipient mice. Astonishingly, none of the recipient mice who underwent IL-21 treatment developed graft-versus-host disease (GVHD) in the MHC-matched allogeneic setting while the graft-versus-tumor (GVT) effect was strongly retained. Inhibition of GVHD onset could also be attributed to the enhanced generation of regulatory B cells (B10) observed in the IL-21, but not PBS, recipient mice. We also tested the thymopoiesis-stimulating property of human IL-21 in NSG mice transplanted with cord blood (CB) and found significant improvement in de novo human CD3+ T cell development.
Conclusions: In sum, our study indicates that IL-21 represents a new class of unforeseen thymopoietin capable of restoring thymic function following BMT.
Keywords: Graft-versus-host disease; Graft-versus-tumor; Hematopoietic stem cells; Humanized mice; IL-21; Regulatory B cells; Spleen; Thymopoiesis.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

