Quantitative proteomics in lung cancer
- PMID: 28615068
- PMCID: PMC5470322
- DOI: 10.1186/s12929-017-0343-y
Quantitative proteomics in lung cancer
Abstract
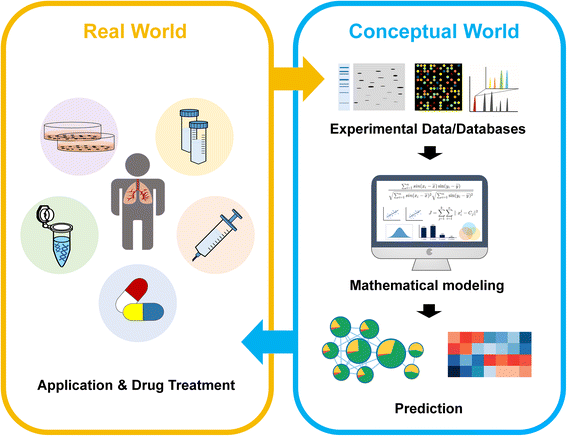
Lung cancer is the most common cause of cancer-related death worldwide, less than 7% of patients survive 10 years following diagnosis across all stages of lung cancer. Late stage of diagnosis and lack of effective and personalized medicine reflect the need for a better understanding of the mechanisms that underlie lung cancer progression. Quantitative proteomics provides the relative different protein abundance in normal and cancer patients which offers the information for molecular interactions, signaling pathways, and biomarker identification. Here we introduce both theoretical and practical applications in the use of quantitative proteomics approaches, with principles of current technologies and methodologies including gel-based, label free, stable isotope labeling as well as targeted proteomics. Predictive markers of drug resistance, candidate biomarkers for diagnosis, and prognostic markers in lung cancer have also been discovered and analyzed by quantitative proteomic analysis. Moreover, construction of protein networks enables to provide an opportunity to interpret disease pathway and improve our understanding in cancer therapeutic strategies, allowing the discovery of molecular markers and new therapeutic targets for lung cancer.
Keywords: Biomarkers; Drug targets; Functional network; Lung cancer; Quantitative proteomics.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

