A Conserved Leucine Zipper Motif in Gammaherpesvirus ORF52 Is Critical for Distinct Microtubule Rearrangements
- PMID: 28615210
- PMCID: PMC5553167
- DOI: 10.1128/JVI.00304-17
A Conserved Leucine Zipper Motif in Gammaherpesvirus ORF52 Is Critical for Distinct Microtubule Rearrangements
Abstract
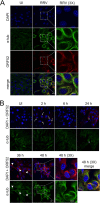
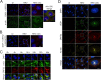
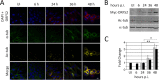
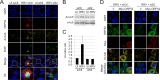
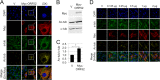
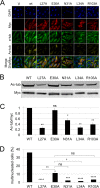
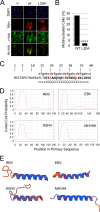
Productive viral infection often depends on the manipulation of the cytoskeleton. Herpesviruses, including rhesus monkey rhadinovirus (RRV) and its close homolog, the oncogenic human gammaherpesvirus Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV8), exploit microtubule (MT)-based retrograde transport to deliver their genomes to the nucleus. Subsequently, during the lytic phase of the life cycle, the maturing viral particles undergo orchestrated translocation to specialized regions within the cytoplasm, leading to tegumentation, secondary envelopment, and then egress. As a result, we hypothesized that RRV might induce changes in the cytoskeleton at both early and late stages of infection. Using confocal imaging, we found that RRV infection led to the thickening and acetylation of MTs emanating from the MT-organizing center (MTOC) shortly after viral entry and more pronounced and diffuse MT reorganization during peak stages of lytic gene expression and virion production. We subsequently identified open reading frame 52 (ORF52), a multifunctional and abundant tegument protein, as being the only virally encoded component responsible for these cytoskeletal changes. Mutational and modeling analyses indicated that an evolutionarily conserved, truncated leucine zipper motif near the N terminus as well as a strictly conserved arginine residue toward the C terminus of ORF52 play critical roles in its ability to rearrange the architecture of the MT cytoskeleton. Taken together, our findings combined with data from previous studies describing diverse roles for ORF52 suggest that it likely binds to different cellular components, thereby allowing context-dependent modulation of function.IMPORTANCE A thorough understanding of the processes governing viral infection includes knowledge of how viruses manipulate their intracellular milieu, including the cytoskeleton. Altering the dynamics of actin or MT polymerization, for example, is a common strategy employed by viruses to ensure efficient entry, maturation, and egress as well as the avoidance of antiviral defenses through the sequestration of key cellular factors. We found that infection with RRV, a homolog of the human pathogen KSHV, led to perinuclear wrapping by acetylated MT bundles and identified ORF52 as the viral protein underlying these changes. Remarkably, incoming virions were able to supply sufficient ORF52 to induce MT thickening and acetylation near the MTOC, potentially aiding in the delivery viral genomes to the nucleus. Although the function of MT alterations during late stages of infection requires further study, ORF52 shares functional and structural similarities with alphaherpesvirus VP22, underscoring the evolutionary importance of MT cytoskeletal manipulations for this virus family.
Keywords: HHV8; Kaposi's sarcoma-associated herpesvirus; RRV; coiled coil; leucine zipper; microtubule-associated protein; rhesus monkey rhadinovirus; tegument.
Copyright © 2017 Loftus et al.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

