Ormeloxifene Suppresses Prostate Tumor Growth and Metastatic Phenotypes via Inhibition of Oncogenic β-catenin Signaling and EMT Progression
- PMID: 28615299
- PMCID: PMC5774234
- DOI: 10.1158/1535-7163.MCT-17-0157
Ormeloxifene Suppresses Prostate Tumor Growth and Metastatic Phenotypes via Inhibition of Oncogenic β-catenin Signaling and EMT Progression
Abstract
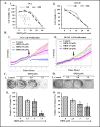
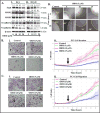
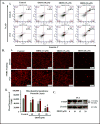
Ormeloxifene is a clinically approved selective estrogen receptor modulator, which has also shown excellent anticancer activity, thus it can be an ideal repurposing pharmacophore. Herein, we report therapeutic effects of ormeloxifene on prostate cancer and elucidate a novel molecular mechanism of its anticancer activity. Ormeloxifene treatment inhibited epithelial-to-mesenchymal transition (EMT) process as evident by repression of N-cadherin, Slug, Snail, vimentin, MMPs (MMP2 and MMP3), β-catenin/TCF-4 transcriptional activity, and induced the expression of pGSK3β. In molecular docking analysis, ormeloxifene showed proficient docking with β-catenin and GSK3β. In addition, ormeloxifene induced apoptosis, inhibited growth and metastatic potential of prostate cancer cells and arrested cell cycle in G0-G1 phase via modulation of cell-cycle regulatory proteins (inhibition of Mcl-1, cyclin D1, and CDK4 and induction of p21 and p27). In functional assays, ormeloxifene remarkably reduced tumorigenic, migratory, and invasive potential of prostate cancer cells. In addition, ormeloxifene treatment significantly (P < 0.01) regressed the prostate tumor growth in the xenograft mouse model while administered through intraperitoneal route (250 μg/mouse, three times a week). These molecular effects of ormeloxifene were also observed in excised tumor tissues as shown by immunohistochemistry analysis. Our results, for the first time, demonstrate repurposing potential of ormeloxifene as an anticancer drug for the treatment of advanced stage metastatic prostate cancer through a novel molecular mechanism involving β-catenin and EMT pathway. Mol Cancer Ther; 16(10); 2267-80. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures



Similar articles
-
Suppression of Akt1-β-catenin pathway in advanced prostate cancer promotes TGFβ1-mediated epithelial to mesenchymal transition and metastasis.Cancer Lett. 2017 Aug 28;402:177-189. doi: 10.1016/j.canlet.2017.05.028. Epub 2017 Jun 7. Cancer Lett. 2017. PMID: 28602980 Free PMC article.
-
P21 activated kinase-1 mediates transforming growth factor β1-induced prostate cancer cell epithelial to mesenchymal transition.Biochim Biophys Acta. 2015 May;1853(5):1229-39. doi: 10.1016/j.bbamcr.2015.02.023. Epub 2015 Mar 6. Biochim Biophys Acta. 2015. PMID: 25746720 Free PMC article.
-
Anti-EMT properties of CoQ0 attributed to PI3K/AKT/NFKB/MMP-9 signaling pathway through ROS-mediated apoptosis.J Exp Clin Cancer Res. 2019 May 8;38(1):186. doi: 10.1186/s13046-019-1196-x. J Exp Clin Cancer Res. 2019. PMID: 31068208 Free PMC article.
-
Epithelial mesenchymal transition and resistance in endocrine-related cancers.Biochim Biophys Acta Mol Cell Res. 2019 Sep;1866(9):1368-1375. doi: 10.1016/j.bbamcr.2019.05.003. Epub 2019 May 18. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31108117 Review.
-
Anti-cancer potential of a novel SERM ormeloxifene.Curr Med Chem. 2013;20(33):4177-84. doi: 10.2174/09298673113209990197. Curr Med Chem. 2013. PMID: 23895678 Free PMC article. Review.
Cited by
-
Pharmacological Efficacy of Repurposing Drugs in the Treatment of Prostate Cancer.Int J Mol Sci. 2023 Feb 19;24(4):4154. doi: 10.3390/ijms24044154. Int J Mol Sci. 2023. PMID: 36835564 Free PMC article. Review.
-
Next-generation paclitaxel-nanoparticle formulation for pancreatic cancer treatment.Nanomedicine. 2019 Aug;20:102027. doi: 10.1016/j.nano.2019.102027. Epub 2019 Jun 4. Nanomedicine. 2019. PMID: 31170509 Free PMC article.
-
PaSTe. Blockade of the Lipid Phenotype of Prostate Cancer as Metabolic Therapy: A Theoretical Proposal.Curr Med Chem. 2024;31(22):3265-3285. doi: 10.2174/0929867330666230607104441. Curr Med Chem. 2024. PMID: 37287286 Review.
-
Reprogramming of pancreatic adenocarcinoma immunosurveillance by a microbial probiotic siderophore.Commun Biol. 2022 Nov 4;5(1):1181. doi: 10.1038/s42003-022-04102-4. Commun Biol. 2022. PMID: 36333531 Free PMC article.
-
A triphenylethylene nonsteroidal SERM attenuates cervical cancer growth.Sci Rep. 2019 Jul 29;9(1):10917. doi: 10.1038/s41598-019-46680-0. Sci Rep. 2019. PMID: 31358785 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

