Prevention and reversal of latent sensitization of dorsal horn neurons by glial blockers in a model of low back pain in male rats
- PMID: 28615336
- PMCID: PMC5626892
- DOI: 10.1152/jn.00680.2016
Prevention and reversal of latent sensitization of dorsal horn neurons by glial blockers in a model of low back pain in male rats
Abstract
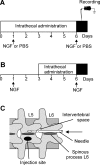
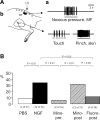
In an animal model of nonspecific low back pain, recordings from dorsal horn neurons were made to investigate the influence of glial cells in the central sensitization process. To induce a latent sensitization of the neurons, nerve growth factor (NGF) was injected into the multifidus muscle; the manifest sensitization to a second NGF injection 5 days later was used as a read-out. The sensitization manifested in increased resting activity and in an increased proportion of neurons responding to stimulation of deep somatic tissues. To block microglial activation, minocycline was continuously administered intrathecally starting 1 day before or 2 days after the first NGF injection. The glia inhibitor fluorocitrate that also blocks astrocyte activation was administrated 2 days after the first injection. Minocycline applied before the first NGF injection reduced the manifest sensitization after the second NGF injection to control values. The proportion of neurons responsive to stimulation of deep tissues was reduced from 50% to 17.7% (P < 0.01). No significant changes occurred when minocycline was applied after the first injection. In contrast, fluorocitrate administrated after the first NGF injection reduced significantly the proportion of neurons with deep input (15.8%, P < 0.01). A block of glia activation had no significant effect on the increased resting activity. The data suggest that blocking microglial activation prevented the NGF-induced latent spinal sensitization, whereas blocking astrocyte activation reversed it. The induction of spinal neuronal sensitization in this pain model appears to depend on microglia activation, whereas its maintenance is regulated by activated astrocytes.NEW & NOTEWORTHY Activated microglia and astrocytes mediate the latent sensitization induced by nerve growth factor in dorsal horn neurons that receive input from deep tissues of the low back. These processes may contribute to nonspecific low back pain.
Keywords: electrophysiology; glial cell activation; latent sensitization; nerve growth factor; nonspecific low back pain.
Copyright © 2017 the American Physiological Society.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

