Donor pulmonary intravascular nonclassical monocytes recruit recipient neutrophils and mediate primary lung allograft dysfunction
- PMID: 28615357
- PMCID: PMC5568853
- DOI: 10.1126/scitranslmed.aal4508
Donor pulmonary intravascular nonclassical monocytes recruit recipient neutrophils and mediate primary lung allograft dysfunction
Abstract
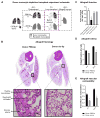
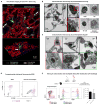
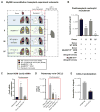
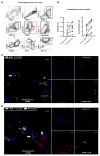
Primary graft dysfunction is the predominant driver of mortality and graft loss after lung transplantation. Recruitment of neutrophils as a result of ischemia-reperfusion injury is thought to cause primary graft dysfunction; however, the mechanisms that regulate neutrophil influx into the injured lung are incompletely understood. We found that donor-derived intravascular nonclassical monocytes (NCMs) are retained in human and murine donor lungs used in transplantation and can be visualized at sites of endothelial injury after reperfusion. When NCMs in the donor lungs were depleted, either pharmacologically or genetically, neutrophil influx and lung graft injury were attenuated in both allogeneic and syngeneic models. Similar protection was observed when the patrolling function of donor NCMs was impaired by deletion of the fractalkine receptor CX3CR1. Unbiased transcriptomic profiling revealed up-regulation of MyD88 pathway genes and a key neutrophil chemoattractant, CXCL2, in donor-derived NCMs after reperfusion. Reconstitution of NCM-depleted donor lungs with wild-type but not MyD88-deficient NCMs rescued neutrophil migration. Donor NCMs, through MyD88 signaling, were responsible for CXCL2 production in the allograft and neutralization of CXCL2 attenuated neutrophil influx. These findings suggest that therapies to deplete or inhibit NCMs in donor lung might ameliorate primary graft dysfunction with minimal toxicity to the recipient.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures




References
-
- Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D ISHLT Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: Definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl. 2005;24:1454–1459. - PubMed
-
- Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, Patterson GA, Trulock EP, Hachem RR. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

