Iron transport proteins: Gateways of cellular and systemic iron homeostasis
- PMID: 28615441
- PMCID: PMC5546014
- DOI: 10.1074/jbc.R117.786632
Iron transport proteins: Gateways of cellular and systemic iron homeostasis
Abstract
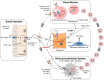
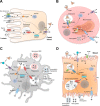
Cellular iron homeostasis is maintained by iron and heme transport proteins that work in concert with ferrireductases, ferroxidases, and chaperones to direct the movement of iron into, within, and out of cells. Systemic iron homeostasis is regulated by the liver-derived peptide hormone, hepcidin. The interface between cellular and systemic iron homeostasis is readily observed in the highly dynamic iron handling of four main cell types: duodenal enterocytes, erythrocyte precursors, macrophages, and hepatocytes. This review provides an overview of how these cell types handle iron, highlighting how iron and heme transporters mediate the exchange and distribution of body iron in health and disease.
Keywords: bone marrow; heme; hepatocyte; iron; macrophage.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The author declares that he has no conflicts of interest with the contents of this article
Figures
References
-
- Gunshin H., Mackenzie B., Berger U. V., Gunshin Y., Romero M. F., Boron W. F., Nussberger S., Gollan J. L., and Hediger M. A. (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482–488 - PubMed
-
- Centers for Disease Control and Prevention (CDC) (2002) Iron deficiency–United States, 1999–2000. MMWR 51, 897–899 - PubMed
-
- Guralnik J. M., Eisenstaedt R. S., Ferrucci L., Klein H. G., and Woodman R. C. (2004) Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 104, 2263–2268 - PubMed
-
- Feder J. N., Gnirke A., Thomas W., Tsuchihashi Z., Ruddy D. A., Basava A., Dormishian F., Domingo R. Jr., Ellis M. C., Fullan A., Hinton L. M., Jones N. L., Kimmel B. E., Kronmal G. S., Lauer P., et al. (1996) A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat. Genet. 13, 399–408 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

