Iron homeostasis: An anthropocentric perspective
- PMID: 28615456
- PMCID: PMC5546013
- DOI: 10.1074/jbc.R117.781823
Iron homeostasis: An anthropocentric perspective
Abstract
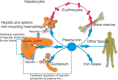
The regulation of iron metabolism in biological systems centers on providing adequate iron for cellular function while limiting iron toxicity. Because mammals cannot excrete iron, mechanisms have evolved to control iron acquisition, storage, and distribution at both systemic and cellular levels. Hepcidin, the master regulator of iron homeostasis, controls iron flows into plasma through inhibition of the only known mammalian cellular iron exporter ferroportin. Hepcidin is feedback-regulated by iron status and strongly modulated by inflammation and erythropoietic demand. This review highlights recent advances that have changed our understanding of iron metabolism and its regulation.
Keywords: erythropoiesis; hepatocyte; infection; inflammation; iron metabolism.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Knutson M. D., Walter P. B., Ames B. N., and Viteri F. E. (2000) Both iron deficiency and daily iron supplements increase lipid peroxidation in rats. J. Nutr. 130, 621–628 - PubMed
-
- Pinilla-Tenas J. J., Sparkman B. K., Shawki A., Illing A. C., Mitchell C. J., Zhao N., Liuzzi J. P., Cousins R. J., Knutson M. D., and Mackenzie B. (2011) Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am. J. Physiol. Cell Physiol. 301, C862–C871 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

