Deconvoluting AMP-activated protein kinase (AMPK) adenine nucleotide binding and sensing
- PMID: 28615457
- PMCID: PMC5535039
- DOI: 10.1074/jbc.M117.793018
Deconvoluting AMP-activated protein kinase (AMPK) adenine nucleotide binding and sensing
Abstract
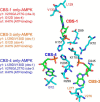
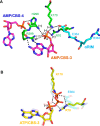
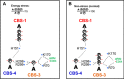
AMP-activated protein kinase (AMPK) is a central cellular energy sensor that adapts metabolism and growth to the energy state of the cell. AMPK senses the ratio of adenine nucleotides (adenylate energy charge) by competitive binding of AMP, ADP, and ATP to three sites (CBS1, CBS3, and CBS4) in its γ-subunit. Because these three binding sites are functionally interconnected, it remains unclear how nucleotides bind to individual sites, which nucleotides occupy each site under physiological conditions, and how binding to one site affects binding to the other sites. Here, we comprehensively analyze nucleotide binding to wild-type and mutant AMPK protein complexes by quantitative competition assays and by hydrogen-deuterium exchange MS. We also demonstrate that NADPH, in addition to the known AMPK ligand NADH, directly and competitively binds AMPK at the AMP-sensing CBS3 site. Our findings reveal how AMP binding to one site affects the conformation and adenine nucleotide binding at the other two sites and establish CBS3, and not CBS1, as the high affinity exchangeable AMP/ADP/ATP-binding site. We further show that AMP binding at CBS4 increases AMP binding at CBS3 by 2 orders of magnitude and reverses the AMP/ATP preference of CBS3. Together, these results illustrate how the three CBS sites collaborate to enable highly sensitive detection of cellular energy states to maintain the tight ATP homeostastis required for cellular metabolism.
Keywords: AMP; AMP-activated kinase (AMPK); ATP; CBS; HDX-MS; NADPH; energy metabolism; protein kinase.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures









References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

