High drug-loading nanomedicines: progress, current status, and prospects
- PMID: 28615938
- PMCID: PMC5459982
- DOI: 10.2147/IJN.S132780
High drug-loading nanomedicines: progress, current status, and prospects
Abstract
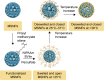
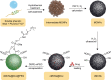
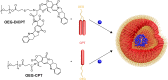
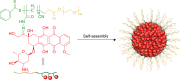
Drug molecules transformed into nanoparticles or endowed with nanostructures with or without the aid of carrier materials are referred to as "nanomedicines" and can overcome some inherent drawbacks of free drugs, such as poor water solubility, high drug dosage, and short drug half-life in vivo. However, most of the existing nanomedicines possess the drawback of low drug-loading (generally less than 10%) associated with more carrier materials. For intravenous administration, the extensive use of carrier materials might cause systemic toxicity and impose an extra burden of degradation, metabolism, and excretion of the materials for patients. Therefore, on the premise of guaranteeing therapeutic effect and function, reducing or avoiding the use of carrier materials is a promising alternative approach to solve these problems. Recently, high drug-loading nanomedicines, which have a drug-loading content higher than 10%, are attracting increasing interest. According to the fabrication strategies of nanomedicines, high drug-loading nanomedicines are divided into four main classes: nanomedicines with inert porous material as carrier, nanomedicines with drug as part of carrier, carrier-free nanomedicines, and nanomedicines following niche and complex strategies. To date, most of the existing high drug-loading nanomedicines belong to the first class, and few research studies have focused on other classes. In this review, we investigate the research status of high drug-loading nanomedicines and discuss the features of their fabrication strategies and optimum proposal in detail. We also point out deficiencies and developing direction of high drug-loading nanomedicines. We envision that high drug-loading nanomedicines will occupy an important position in the field of drug-delivery systems, and hope that novel perspectives will be proposed for the development of high drug-loading nanomedicines.
Keywords: fabrication strategy; high drug loading; nanocarriers; nanomedicines; optimum proposal.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures






References
-
- Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420(1):1–10. - PubMed
-
- Liggins RT, Burt HM. Polyether-polyester diblock copolymers for the preparation of paclitaxel loaded polymeric micelle formulations. Adv Drug Deliv Rev. 2002;54(2):191–202. - PubMed
-
- Du WT, Hong L, Yao TW, et al. Synthesis and evaluation of water-soluble docetaxel prodrugs-docetaxel esters of malic acid. Bioorg Med Chem. 2007;15(18):6323–6330. - PubMed
-
- Luke DR, Kasiske BL, Matzke GR, Awni WM, Keane WF. Effects of cyclosporine on the isolated perfused rat kidney. Transplantation. 1987;43(6):795–799. - PubMed
-
- Onetto N, Canetta R, Winograd B, et al. Overview of Taxol safety. J Natl Cancer Inst Monogr. 1993;(15):131–139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

