Arbaclofen in fragile X syndrome: results of phase 3 trials
- PMID: 28616094
- PMCID: PMC5467054
- DOI: 10.1186/s11689-016-9181-6
Arbaclofen in fragile X syndrome: results of phase 3 trials
Abstract
Background: Arbaclofen improved multiple abnormal phenotypes in animal models of fragile X syndrome (FXS) and showed promising results in a phase 2 clinical study. The objective of the study is to determine safety and efficacy of arbaclofen for social avoidance in FXS.
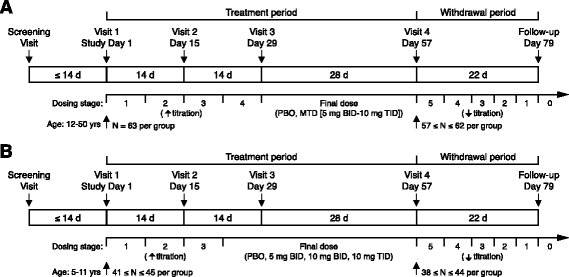
Methods: Two phase 3 placebo-controlled trials were conducted, a flexible dose trial in subjects age 12-50 (209FX301, adolescent/adult study) and a fixed dose trial in subjects age 5-11 (209FX302, child study). The primary endpoint for both trials was the Social Avoidance subscale of the Aberrant Behavior Checklist-Community Edition, FXS-specific (ABC-CFX). Secondary outcomes included other ABC-CFX subscale scores, Clinical Global Impression-Improvement (CGI-I), Clinical Global Impression-Severity (CGI-S), and Vineland Adaptive Behavior Scales, Second Edition (Vineland-II) Socialization domain score.
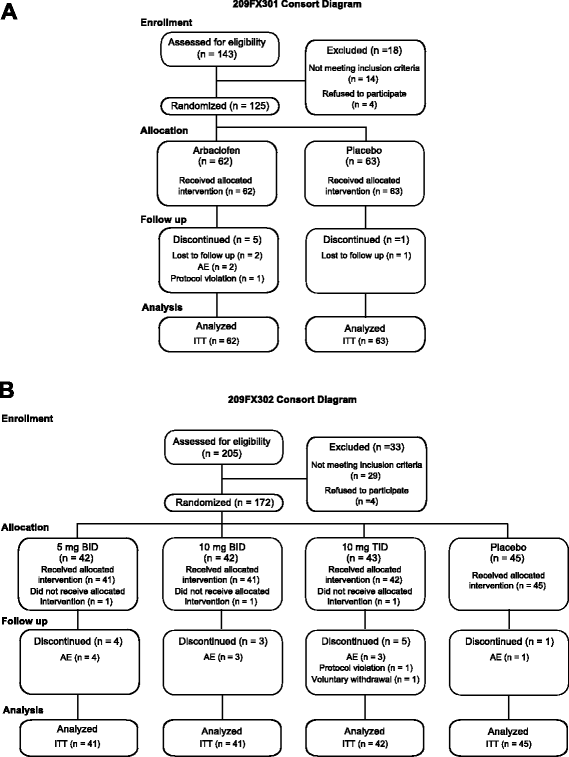
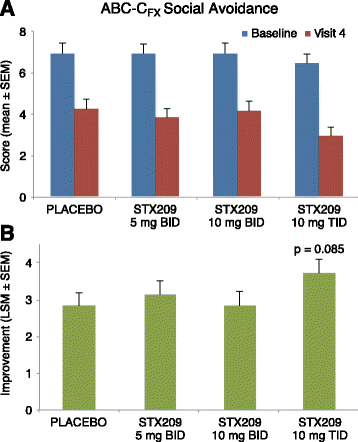
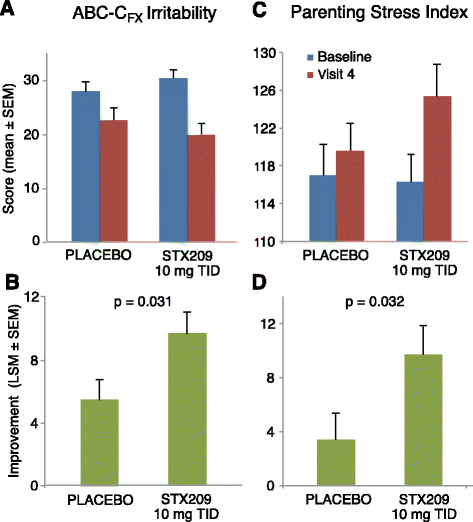
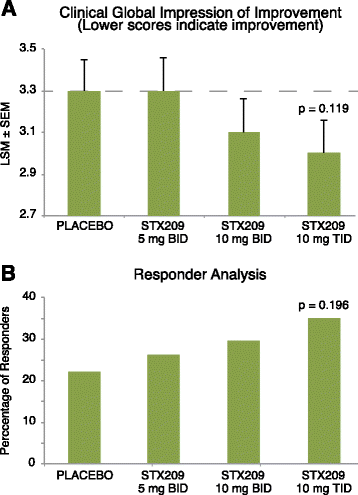
Results: A total 119 of 125 randomized subjects completed the adolescent/adult study (n = 57 arbaclofen, 62 placebo) and 159/172 completed the child study (arbaclofen 5 BID n = 38; 10 BID n = 39; 10 TID n = 38; placebo n = 44). There were no serious adverse events (AEs); the most common AEs included somatic (headache, vomiting, nausea), neurobehavioral (irritability/agitation, anxiety, hyperactivity), decreased appetite, and infectious conditions, many of which were also common on placebo. In the combined studies, there were 13 discontinuations (n = 12 arbaclofen, 1 placebo) due to AEs (all neurobehavioral). The adolescent/adult study did not show benefit for arbaclofen over placebo for any measure. In the child study, the highest dose group showed benefit over placebo on the ABC-CFX Irritability subscale (p = 0.03) and Parenting Stress Index (PSI, p = 0.03) and trends toward benefit on the ABC-CFX Social Avoidance and Hyperactivity subscales (both p < 0.1) and CGI-I (p = 0.119). Effect size in the highest dose group was similar to effect sizes for FDA-approved serotonin reuptake inhibitors (SSRIs).
Conclusions: Arbaclofen did not meet the primary outcome of improved social avoidance in FXS in either study. Data from secondary measures in the child study suggests younger patients may derive benefit, but additional studies with a larger cohort on higher doses would be required to confirm this finding. The reported studies illustrate the challenges but represent a significant step forward in translating targeted treatments from preclinical models to clinical trials in humans with FXS.
Keywords: Arbaclofen; FMR1; Fragile X syndrome; GABA agonist; Neurodevelopmental disorder; Targeted treatment.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

