A family of cis-macrocyclic diphosphines: modular, stereoselective synthesis and application in catalytic CO2/ethylene coupling
- PMID: 28616145
- PMCID: PMC5460601
- DOI: 10.1039/c6sc03614g
A family of cis-macrocyclic diphosphines: modular, stereoselective synthesis and application in catalytic CO2/ethylene coupling
Abstract
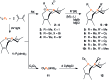
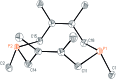
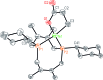
A family of cis-macrocyclic diphosphines was prepared in just three steps from white phosphorus and commercial materials using a modular synthetic approach. Alkylation of bicyclic diphosphane 3,4,8,9-tetramethyl-1,6-diphosphabicyclo(4.4.0)deca-3,8-diene, or P2(dmb)2, produced phosphino-phosphonium salts [R-P2(dmb)2]X, where R is methyl, benzyl and isobutyl, in yields of 90-96%. Treatment of these salts with organolithium or Grignard reagents yielded symmetric and unsymmetric macrocyclic diphosphines of the form cis-1-R-6-R'-3,4,8,9-tetramethyl-2,5,7,10-tetrahydro-1,6-DiPhospheCine, or R,R'-DPC, in which R' is methyl, cyclohexyl, phenyl or mesityl, in yields of 46-94%. Alternatively, symmetric diphosphine Cy2-DPC was synthesized in 74% yield from the dichlorodiphosphine Cl2P2(dmb)2. As a first application, these cis-macrocyclic diphosphines were used as ligands in the nickel-catalyzed synthesis of acrylate from CO2 and ethylene, for which they showed promising catalytic activity.
Figures





Similar articles
-
Synthesis, Characterization, and Catalytic Activity of Ni(II) Alkyl Complexes Supported by Pyrrole-Diphosphine Ligands.Organometallics. 2013 Aug 26;32(16):4656-4663. doi: 10.1021/om400630q. Organometallics. 2013. PMID: 24567662 Free PMC article.
-
The autoxidation and proton dissociation constants of tertiary diphosphines: relevance to biological activity.J Inorg Biochem. 1987 Nov;31(3):197-209. doi: 10.1016/0162-0134(87)80005-3. J Inorg Biochem. 1987. PMID: 2828542
-
Functionalization reactions characteristic of a robust bicyclic diphosphane framework.Inorg Chem. 2013 Aug 5;52(15):8851-64. doi: 10.1021/ic401052a. Epub 2013 Jul 10. Inorg Chem. 2013. PMID: 23841641
-
Chiral diphosphine and monodentate phosphorus ligands on a spiro scaffold for transition-metal-catalyzed asymmetric reactions.Acc Chem Res. 2008 May;41(5):581-93. doi: 10.1021/ar700137z. Epub 2008 Mar 1. Acc Chem Res. 2008. PMID: 18311931
-
A strategy for the stereoselective synthesis of unsymmetric atropisomeric ligands: preparation of NAPhePHOS, a new biaryl diphosphine.Chemistry. 2002 Aug 2;8(15):3327-30. doi: 10.1002/1521-3765(20020802)8:15<3327::AID-CHEM3327>3.0.CO;2-F. Chemistry. 2002. PMID: 12203313
Cited by
-
Functionalization of P4 through Direct P-C Bond Formation.Chemistry. 2017 Sep 4;23(49):11738-11746. doi: 10.1002/chem.201702067. Epub 2017 Jul 27. Chemistry. 2017. PMID: 28497639 Free PMC article. Review.
References
-
- Hartwig J., Organotransition Metal Chemistry: From Bonding to Catalysis, University Science books, 1st edn, 2010.
-
- Chan T. H., Ong B. S. J. Org. Chem. 1974;39:1748–1752.
- Ignat'eva S. N., Balueva A. S., Karasik A. A., Kulikov D. V., Kozlov A. V., Latypov S. K., Lönnecke P., Hey-Hawkins E., Sinyashin O. G. Russ. Chem. Bull. 2007;56:1828–1837.
- Horner L., Walach P., Kunz H. Phosphorus, Sulfur Silicon Relat. Elem. 1978;5:171–184.
-
- Bauer E. B., Ruwwe J., Martin-Alvarez J. M., Peters T. B., Bohling J. C., Hampel F. A., Szafert S., Lis T., Gladysz J. A. Chem. Commun. 2000:2261–2262.
- Shima T., Bauer E. B., Hampel F., Gladysz J. A. Dalton Trans. 2004:1012–1028. - PubMed
-
- Alder R. W., Ganter C., Harris C. J., Orpen A. G. J. Chem. Soc., Chem. Commun. 1992:1170–1172.
-
- Alder R. W., Ellis D. D., Hogg J. K., Martín A., Orpen A. G., Taylor P. N. Chem. Commun. 1996:537–538.
- Alder R. W., Ganter C., Gil M., Gleiter R., Harris C. J., Harris S. E., Lange H., Orpen A. G., Taylor P. N. J. Chem. Soc., Perkin Trans. 1. 1998:1643–1656.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

