Liver segmentation: indications, techniques and future directions
- PMID: 28616760
- PMCID: PMC5519497
- DOI: 10.1007/s13244-017-0558-1
Liver segmentation: indications, techniques and future directions
Abstract
Objectives: Liver volumetry has emerged as an important tool in clinical practice. Liver volume is assessed primarily via organ segmentation of computed tomography (CT) and magnetic resonance imaging (MRI) images. The goal of this paper is to provide an accessible overview of liver segmentation targeted at radiologists and other healthcare professionals.
Methods: Using images from CT and MRI, this paper reviews the indications for liver segmentation, technical approaches used in segmentation software and the developing roles of liver segmentation in clinical practice.
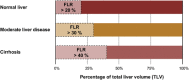
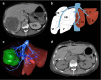
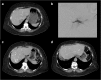
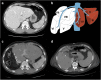
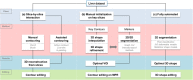
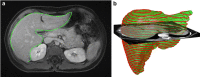
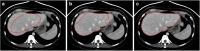
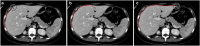
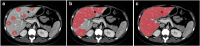
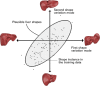
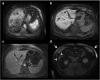
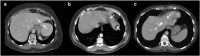
Results: Liver segmentation for volumetric assessment is indicated prior to major hepatectomy, portal vein embolisation, associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) and transplant. Segmentation software can be categorised according to amount of user input involved: manual, semi-automated and fully automated. Manual segmentation is considered the "gold standard" in clinical practice and research, but is tedious and time-consuming. Increasingly automated segmentation approaches are more robust, but may suffer from certain segmentation pitfalls. Emerging applications of segmentation include surgical planning and integration with MRI-based biomarkers.
Conclusions: Liver segmentation has multiple clinical applications and is expanding in scope. Clinicians can employ semi-automated or fully automated segmentation options to more efficiently integrate volumetry into clinical practice.
Teaching points: • Liver volume is assessed via organ segmentation on CT and MRI examinations. • Liver segmentation is used for volume assessment prior to major hepatic procedures. • Segmentation approaches may be categorised according to the amount of user input involved. • Emerging applications include surgical planning and integration with MRI-based biomarkers.
Keywords: Automated; Computed tomography; Liver; Magnetic resonance imaging; Segmentation; Volumetry.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

