Tramadol for neuropathic pain in adults
- PMID: 28616956
- PMCID: PMC6481580
- DOI: 10.1002/14651858.CD003726.pub4
Tramadol for neuropathic pain in adults
Abstract
Background: This review is an update of a review of tramadol for neuropathic pain, published in 2006; updating was to bring the review in line with current standards. Neuropathic pain, which is caused by a lesion or disease affecting the somatosensory system, may be central or peripheral in origin. Peripheral neuropathic pain often includes symptoms such as burning or shooting sensations, abnormal sensitivity to normally painless stimuli, or an increased sensitivity to normally painful stimuli. Neuropathic pain is a common symptom in many diseases of the peripheral nervous system.
Objectives: To assess the analgesic efficacy of tramadol compared with placebo or other active interventions for chronic neuropathic pain in adults, and the adverse events associated with its use in clinical trials.
Search methods: We searched CENTRAL, MEDLINE, and Embase for randomised controlled trials from inception to January 2017. We also searched the reference lists of retrieved studies and reviews, and online clinical trial registries.
Selection criteria: We included randomised, double-blind trials of two weeks' duration or longer, comparing tramadol (any route of administration) with placebo or another active treatment for neuropathic pain, with subjective pain assessment by the participant.
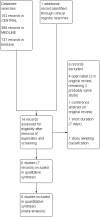
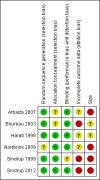
Data collection and analysis: Two review authors independently extracted data and assessed trial quality and potential bias. Primary outcomes were participants with substantial pain relief (at least 50% pain relief over baseline or very much improved on Patient Global Impression of Change scale (PGIC)), or moderate pain relief (at least 30% pain relief over baseline or much or very much improved on PGIC). Where pooled analysis was possible, we used dichotomous data to calculate risk ratio (RR) and number needed to treat for an additional beneficial outcome (NNT) or harmful outcome (NNH), using standard methods. We assessed the quality of the evidence using GRADE and created 'Summary of findings' tables.
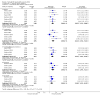
Main results: We identified six randomised, double-blind studies involving 438 participants with suitably characterised neuropathic pain. In each, tramadol was started at a dose of about 100 mg daily and increased over one to two weeks to a maximum of 400 mg daily or the maximum tolerated dose, and then maintained for the remainder of the study. Participants had experienced moderate or severe neuropathic pain for at least three months due to cancer, cancer treatment, postherpetic neuralgia, peripheral diabetic neuropathy, spinal cord injury, or polyneuropathy. The mean age was 50 to 67 years with approximately equal numbers of men and women. Exclusions were typically people with other significant comorbidity or pain from other causes. Study duration for treatments was four to six weeks, and two studies had a cross-over design.Not all studies reported all the outcomes of interest, and there were limited data for pain outcomes. At least 50% pain intensity reduction was reported in three studies (265 participants, 110 events). Using a random-effects analysis, 70/132 (53%) had at least 50% pain relief with tramadol, and 40/133 (30%) with placebo; the risk ratio (RR) was 2.2 (95% confidence interval (CI) 1.02 to 4.6). The NNT calculated from these data was 4.4 (95% CI 2.9 to 8.8). We downgraded the evidence for this outcome by two levels to low quality because of the small size of studies and of the pooled data set, because there were only 110 actual events, the analysis included different types of neuropathic pain, the studies all had at least one high risk of potential bias, and because of the limited duration of the studies.Participants experienced more adverse events with tramadol than placebo. Report of any adverse event was higher with tramadol (58%) than placebo (34%) (4 studies, 266 participants, 123 events; RR 1.6 (95% CI 1.2 to 2.1); NNH 4.2 (95% CI 2.8 to 8.3)). Adverse event withdrawal was higher with tramadol (16%) than placebo (3%) (6 studies, 485 participants, 45 events; RR 4.1 (95% CI 2.0 to 8.4); NNH 8.2 (95% CI 5.8 to 14)). Only four serious adverse events were reported, without obvious attribution to treatment, and no deaths were reported. We downgraded the evidence for this outcome by two or three levels to low or very low quality because of small study size, because there were few actual events, and because of the limited duration of the studies.
Authors' conclusions: There is only modest information about the use of tramadol in neuropathic pain, coming from small, largely inadequate studies with potential risk of bias. That bias would normally increase the apparent benefits of tramadol. The evidence of benefit from tramadol was of low or very low quality, meaning that it does not provide a reliable indication of the likely effect, and the likelihood is very high that the effect will be substantially different from the estimate in this systematic review.
Conflict of interest statement
RMD: none known
SD: none known
PW: none known
RFB: none known. RFB is a retired specialist pain physician who has managed patients with neuropathic pain.
DA: is a specialist pain physician and manages patients with neuropathic pain. He has received lecture fees from Grünenthal (2014, 2015) and Pfizer (2016).
RAM: RAM has received grant support from Grünenthal relating to individual patient‐level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015). He has received honoraria from Omega Pharma (2016) and Futura Pharma (2016) for providing advice on trial and data analysis methods.
Figures





Update of
-
Tramadol for neuropathic pain.Cochrane Database Syst Rev. 2006 Jul 19;(3):CD003726. doi: 10.1002/14651858.CD003726.pub3. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2017 Jun 15;6:CD003726. doi: 10.1002/14651858.CD003726.pub4. PMID: 16856016 Updated.
References
References to studies included in this review
Arbaiza 2007 {published data only}
-
- Arbaiza D, Vidal O. Tramadol in the treatment of neuropathic cancer pain. A double‐blind, placebo‐controlled study. Clinical Drug Investigation 2007;27(1):75‐83. [PUBMED: 17177582] - PubMed
Boureau 2003 {published data only}
-
- Boureau F, Legallicier P, Kabir‐Ahmadi M. Tramadol in post‐herpetic neuralgia: a randomized, double‐blind, placebo‐controlled trial. Pain 2003;104(1‐2):323‐31. [PUBMED: 12855342] - PubMed
Harati 1998 {published data only}
-
- Harati Y, Gooch C, Swenson M, Edelman S, Greene D, Raskin P, et al. Double‐blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 1998;50(6):1842‐6. [PUBMED: 9633738] - PubMed
Norrbrink 2009 {published data only}
Sindrup 1999 {published data only}
Sindrup 2012 {published data only}
-
- Sindrup SH, Konder R, Lehmann R, Meier T, Winkel M, Ashworth J, et al. Randomized controlled trial of the combined monoaminergic and opioid investigational compound GRT9906 in painful polyneuropathy. European Journal of Pain 2012;16(6):849‐59. [DOI: 10.1002/j.1532-2149.2011.00069.x; PUBMED: 22337471] - DOI - PubMed
References to studies excluded from this review
Ashry 2001 {published data only}
-
- Ashry H. Double‐blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Foot and Ankle Quarterly ‐ The Seminar Journal 2001;14(4):129‐31.
Attal 2001 {published data only}
-
- Attal N. Pharmacologic treatment of neuropathic pain. Acta Neurologica Belgica 2001;101(1):53‐64. - PubMed
Benedetti 1998 {published data only}
-
- Benedetti F, Vighetti S, Amanzio M, Casadio C, Oliaro A, Bergamasco B, et al. Dose‐response relationship of opioids in nociceptive and neuropathic postoperative pain. Pain 1998;74(2‐3):205‐11. [MEDLINE: ] - PubMed
Erdine 1997 {published data only}
-
- Erdine S, Yucel A, Ozyalcin S. Efficacy of tramadol hydrochloride in chronic painful diabetic neuropathy: a double‐blind placebo controlled study. Proceedings of the 8th World Congress on Pain. Seattle: IASP Press, 1997:371.
Gobel 1995 {published data only}
-
- Gobel H, Stadler TH. Treatment of pain due to postherpetic neuralgia with tramadol. Clinical Drug Investigation 1995;10(4):208‐14.
Harati 1999 {published data only}
-
- Harati Y. Tramadol for the treatment of the pain of diabetic neuropathy [Correspondence]. Neurology 1999;52(6):1300. - PubMed
Harati 2000 {published data only}
-
- Harati Y, Gooch C, Swenson M, Edelman SV, Greene D, Raskin P, et al. Maintenance of long‐term effectiveness of tramadol in treatment of the pain of diabetic neuropathy. Journal of Diabetes and its Complications 2000;14(2):65‐70. [PUBMED: 10959067] - PubMed
Herrera Silva 2001 {published data only}
-
- Herrera Silva J. The use of oral opioids in neuropathic pain: development of new tramadol and morphine formulations [Utilidad de los opioides orales en dolor neuropático. Apariciónde nuevas formulaciones de tramadoly morfina]. Revista de la Sociedad Espanola del Dolor 2001;8(Suppl):32‐4.
Leppert 2001 {published data only}
-
- Leppert W. Analgesic efficacy and side effects of oral tramadol and morphine administered orally in the treatment of cancer pain. Nowotwory 2001;51(3):257‐66.
Moulin 1999 {published data only}
-
- Moulin D. Tramadol for the treatment of the pain of diabetic neuropathy [Correspondence]. Neurology 1999;52(6):1301. - PubMed
NCT00610155 {published data only}
-
- NCT00610155. A methodology study of brain imaging of pain‐killers in post‐traumatic neuropathic pain patients [A methodology study to assess the feasibility of using functional magnetic resonance imaging (fMRI) to quantify the effects of analgesic drugs in post‐traumatic neuropathic pain subjects]. clinicaltrials.gov/ct2/show/NCT00610155 (first received 14 January 2008). [CTG: NCT00610155]
Saxena 2013 {published data only}
-
- Nasare NV, Banerjee BD, Deshmukh PS, Mediratta PK, Ahmed RS, Saxena AK, et al. Neuropathic pain symptoms of post herpetic neuralgia patients with CYP2D6 polymorphism undergoing tramadol treatment. International Journal of Pharmaceutical Sciences and Research 2015;6(4):1489‐501. [DOI: 10.13040/IJPSR.0975-8232.6(4).1489-01] - DOI
-
- Saxena AK, Nasare N, Jain S, Dhakate G, Ahmed RS, Bhattacharya SN, et al. A randomized, prospective study of efficacy and safety of oral tramadol in the management of post‐herpetic neuralgia in patients from north India. Pain Practice 2013;13(4):264‐75. [DOI: 10.1111/j.1533-2500.2012.00583.x] - DOI - PubMed
Xiao 2004 {published data only}
-
- Xiao LZ, Zhang DR, Jiang J, Zhang KL, Zhang M, Zhu HQ. Feasibility of transdermal fentanyl for pain relief of herpes zoster and postherpetic neuralgia. Zhongguo Linchuang Kangfu 2004;8(2):216‐7.
References to studies awaiting assessment
Additional references
AlBalawi 2013
Baron 2012
Beakley 2015
-
- Beakley BD, Kaye AM, Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician 2015;18(4):395‐400. [PUBMED: 26218943] - PubMed
Berger 2004
Berger 2009
Berger 2012
Bouhassira 2008
Bozkurt 2005
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive‐‐Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI: 10.1093/ije/dyn188] - DOI - PubMed
Bush 2015
-
- Bush DM. Emergency department visits for drug misuse or abuse involving the pain medication tramadol. The CBHSQ Report. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2013‐.. The CBHSQ, 2015; Vol. May 14. - PubMed
CADTH 2015
-
- Tramadol for the management of pain in adult patients: a review of the clinical effectiveness. Canadian Agency for Drugs and Technologies in Health 2 February 2015; Vol. www.cadth.ca/sites/default/files/pdf/htis/may‐2015/RC0627‐Tramadol‐pain‐... (accessed 9 January 2017). - PubMed
Calvo 2012
Cepeda 2006
Chaparro 2012
Dechartres 2013
Dechartres 2014
Demant 2014
-
- Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double‐blind, placebo‐controlled phenotype‐stratified study. Pain 2014;155(11):2263‐73. [DOI: 10.1016/j.pain.2014.08.014] - DOI - PubMed
Derry 2012
Derry 2013
Derry 2014
Dworkin 2008
Dworkin 2013
Edwards 2002
EPOC 2015
-
- Effective Practice, Organisation of Care (EPOC). 23. Worksheets for preparing a Summary of Findings using GRADE. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2015. Available at: http://epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed 9 January 2017).
Finnerup 2013
Finnerup 2015
Gan 2007
Gaskell 2016
Grond 2004
Gustorff 2008
Guyatt 2013a
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [DOI: 10.1016/j.jclinepi.2012.01.006] - DOI - PubMed
Guyatt 2013b
Hall 2008
Helfert 2015
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffman 2010
Jadad 1996
Jensen 2011
Kalso 2013
Katusic 1991
-
- Katusic S, Williams DB, Beard CM, Bergstralh EJ, Kurland LT. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota 1945‐1984. Neuroepidemiology 1991;10(5‐6):276‐81. [DOI: 10.1159/000110284] - DOI - PubMed
Koopman 2009
L'Abbé 1987
Lintz 1998
-
- Lintz W, Barth H, Osterloh G, Schmidt‐Bothelt E. Pharmacokinetics of tramadol and bioavailability of enteral tramadol formulations. 3rd Communication: suppositories. Arzneimittel‐Forschung 1998;48(9):889‐99. [PUBMED: 9793614] - PubMed
Lunn 2014
Martindale 2016
-
- Martindale: the complete drug reference. Tramadol hydrochloride. www.medicinescomplete.com/mc/ (accessed 9 January 2017). 38th. Pharmaceutical Press, 2016.
McQuay 1996
McQuay 1998
-
- McQuay HJ, Moore RA. An Evidence‐Based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0‐19‐263048‐2]
McQuay 2007
-
- McQuay HJ, Smith LA, Moore RA. Chronic pain. In: Stevens A, Raftery J, Mant J, Simpson S editor(s). Health Care Needs Assessment, 3rd Series. Oxford: Radcliffe Publishing, 2007. [ISBN: 978‐1‐84619‐063‐6]
Moher 2009
Moore 1997
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5]
Moore 2009
Moore 2010a
Moore 2010b
Moore 2010c
Moore 2010d
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] - DOI - PMC - PubMed
Moore 2011a
Moore 2011b
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: 10.1016/j.pain.2010.11.030] - DOI - PubMed
Moore 2012
Moore 2013a
Moore 2013b
Moore 2014a
Moore 2014b
Moore 2014c
Moore 2015a
Moore 2015b
Moore 2015c
NICE 2013
-
- National Institute for Health and Care Excellence (NICE). Neuropathic pain ‐ pharmacological management: the pharmacological management of neuropathic pain in adults in non‐specialist settings, 2013. www.nice.org.uk/guidance/cg173 (accessed 9 January 2017). - PubMed
Nüesch 2010
O'Brien 2010
PaPaS 2012
-
- Cochrane Pain, Palliative and Supportive Care Group (PaPaS) author and referee guidance. papas.cochrane.org/papas‐documents (accessed 9 January 2017).
Radbruch 2013
Rappaport 1994
Reeves 2008
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2015
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Scott 2000
Scott 2006
Stannard 2016
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrolment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.] - DOI - PMC - PubMed
Straube 2010
Sultan 2008
Tassinari 2011
Thorlund 2011
Torrance 2006
Treede 2008
Turner 2013
Van Hecke 2014
Van Hoek 2009
von Hehn 2012
Vos 2012
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: 10.1016/S0140-6736(12)61729-2] - DOI - PMC - PubMed
WHO 2016
-
- World Health Organization (WHO). WHO analgesic ladder. www.who.int/cancer/palliative/painladder/en/ (accessed 9 January 2017).
References to other published versions of this review
Dühmke 2002
Dühmke 2004
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

