Characterization of highly concentrated antibody solution - A toolbox for the description of protein long-term solution stability
- PMID: 28617076
- PMCID: PMC5627599
- DOI: 10.1080/19420862.2017.1338222
Characterization of highly concentrated antibody solution - A toolbox for the description of protein long-term solution stability
Abstract
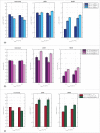
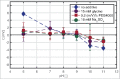
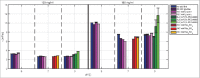
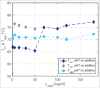
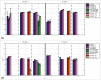
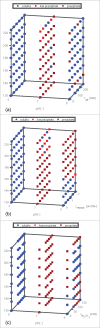
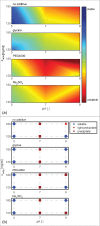
High protein titers are gaining importance in biopharmaceutical industry. A major challenge in the development of highly concentrated mAb solutions is their long-term stability and often incalculable viscosity. The complexity of the molecule itself, as well as the various molecular interactions, make it difficult to describe their solution behavior. To study the formulation stability, long- and short-range interactions and the formation of complex network structures have to be taken into account. For a better understanding of highly concentrated solutions, we combined established and novel analytical tools to characterize the effect of solution properties on the stability of highly concentrated mAb formulations. In this study, monoclonal antibody solutions in a concentration range of 50-200 mg/ml at pH 5-9 with and without glycine, PEG4000, and Na2SO4 were analyzed. To determine the monomer content, analytical size-exclusion chromatography runs were performed. ζ-potential measurements were conducted to analyze the electrophoretic properties in different solutions. The melting and aggregation temperatures were determined with the help of fluorescence and static light scattering measurements. Additionally, rheological measurements were conducted to study the solution viscosity and viscoelastic behavior of the mAb solutions. The so-determined analytical parameters were scored and merged in an analytical toolbox. The resulting scoring was then successfully correlated with long-term storage (40 d of incubation) experiments. Our results indicate that the sensitivity of complex rheological measurements, in combination with the applied techniques, allows reliable statements to be made with respect to the effect of solution properties, such as protein concentration, ionic strength, and pH shift, on the strength of protein-protein interaction and solution colloidal stability.
Keywords: Conformational and colloidal stability; monoclonal antibodies; phase diagram; thermal stability; viscoelasticity; viscosity; zeta-potential.
Figures

References
-
- Rosman Z, Shoenfeld Y, Zandmann-Goddard G. Biologic therapy for autoimmune diseases: An update. BMC Med 2013; 11:88; PMID:23557513; https://doi.org/ 10.1186/1741-7015-11-88 - DOI - PMC - PubMed
-
- Scott A, Wolchok J, Old L. Antibody therapy of cancer. Nat Rev Cancer 2012; 12:14; PMID:22437872; https://doi.org/ 10.1038/nrc3236 - DOI - PubMed
-
- Shire SJ, Shahrokh Z, Liu JUN. Challenges in the development of high protein concentration formulations. J Pharm Sci 2004; 93:1390-402; PMID:15124199; https://doi.org/ 10.1002/jps.20079 - DOI - PubMed
-
- Pindrus M, Shire S, Kelley R, Demeule B, Wong R. Solubility challenges in high concentration monoclonal antibody formulations: Relationship with amino acid sequence and intermolecular interactions. Mol Pharm 2015; 12:3896-907; PMID:26407030; https://doi.org/ 10.1021/acs.molpharmaceut.5b00336 - DOI - PubMed
-
- Leckband D, Sivasankar S. Forces controlling protein interactions: Theory and experiment. Cool Surfaces B Biointerfaces 1999; 14:83-97; https://doi.org/ 10.1016/S0927-7765(99)00027-2 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
