NOX2-Induced Activation of Arginase and Diabetes-Induced Retinal Endothelial Cell Senescence
- PMID: 28617308
- PMCID: PMC5488023
- DOI: 10.3390/antiox6020043
NOX2-Induced Activation of Arginase and Diabetes-Induced Retinal Endothelial Cell Senescence
Abstract
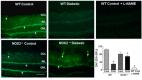
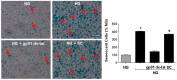
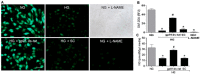
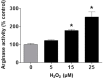
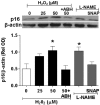
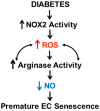
Increases in reactive oxygen species (ROS) and decreases in nitric oxide (NO) have been linked to vascular dysfunction during diabetic retinopathy (DR). Diabetes can reduce NO by increasing ROS and by increasing activity of arginase, which competes with nitric oxide synthase (NOS) for their commons substrate l-arginine. Increased ROS and decreased NO can cause premature endothelial cell (EC) senescence leading to defective vascular repair. We have previously demonstrated the involvement of NADPH oxidase 2 (NOX2)-derived ROS, decreased NO and overactive arginase in DR. Here, we investigated their impact on diabetes-induced EC senescence. Studies using diabetic mice and retinal ECs treated with high glucose or H₂O₂ showed that increases in ROS formation, elevated arginase expression and activity, and decreased NO formation led to premature EC senescence. NOX2 blockade or arginase inhibition prevented these effects. EC senescence was also increased by inhibition of NOS activity and this was prevented by treatment with a NO donor. These results indicate that diabetes/high glucose-induced activation of arginase and decreases in NO bioavailability accelerate EC senescence. NOX2-generated ROS contribute importantly to this process. Blockade of NOX2 or arginase represents a strategy to prevent diabetes-induced premature EC senescence by preserving NO bioavailability.
Keywords: NO; NOS; NOX2/NADPH oxidase 2; arginase 1; diabetic retinopathy; endothelial cell; oxidative stress; retina; senescence associated β-galactosidase.
Conflict of interest statement
The authors declare that there is no conflict of interest associated with this manuscript.
Figures





References
-
- Goldberg M.F. Retinal detachment associated with proliferative retinopathies (sickle cell disease, retrolental fibroplasia and diabetes mellitus) Isr. J. Med. Sci. 1972;8:1447–1457. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

