Coumarin-Tetrapyrrolic Macrocycle Conjugates: Synthesis and Applications
- PMID: 28617340
- PMCID: PMC6152750
- DOI: 10.3390/molecules22060994
Coumarin-Tetrapyrrolic Macrocycle Conjugates: Synthesis and Applications
Abstract
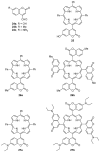
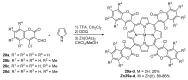
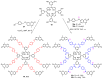
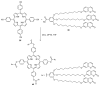
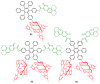
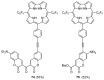
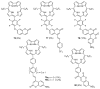
This review covers the synthesis of coumarin-porphyrin, coumarin-phthalocyanine and coumarin-corrole conjugates and their potential applications. While coumarin-phthalocyanine conjugates were obtained almost exclusively by tetramerization of coumarin-functionalized phthalonitriles, coumarin-porphyrin and coumarin-corrole conjugates were prepared by complementary approaches: (a) direct synthesis of the tetrapyrrolic macrocycle using formylcoumarins and pyrrole or (b) by functionalization of the tetrapyrrolic macrocycle. In the last approach a range of reaction types were used, namely 1,3-dipolar cycloadditions, hetero-Diels-Alder, Sonogashira, alkylation or acylation reactions. This is clearly a more versatile approach, leading to a larger diversity of conjugates and allowing the access to conjugates bearing one to up to 16 coumarin units.
Keywords: corroles; coumarins; phthalocyanines; porphyrins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




















Similar articles
-
Synthesis of Porphyrin, Chlorin and Phthalocyanine Derivatives by Azide-Alkyne Click Chemistry.Curr Med Chem. 2015;22(28):3217-54. doi: 10.2174/0929867322666150716115832. Curr Med Chem. 2015. PMID: 26179994 Review.
-
Strategies for Corrole Functionalization.Chem Rev. 2017 Feb 22;117(4):3192-3253. doi: 10.1021/acs.chemrev.6b00476. Epub 2016 Nov 29. Chem Rev. 2017. PMID: 28222602
-
Synthesis, structure, and aromaticity of the nickel(II) complex of pyricorrole, a molecular hybrid of porphyrin and corrole.Inorg Chem. 2012 Mar 19;51(6):3891-5. doi: 10.1021/ic3001043. Epub 2012 Mar 6. Inorg Chem. 2012. PMID: 22394100
-
Synthesis and functionalization of meso-aryl-substituted corroles.J Org Chem. 2001 Jan 26;66(2):550-6. doi: 10.1021/jo005661t. J Org Chem. 2001. PMID: 11429828
-
Peptide-Tetrapyrrole Supramolecular Self-Assemblies: State of the Art.Molecules. 2021 Jan 28;26(3):693. doi: 10.3390/molecules26030693. Molecules. 2021. PMID: 33525730 Free PMC article. Review.
Cited by
-
Anticarin β Inhibits Human Glioma Progression by Suppressing Cancer Stemness via STAT3.Front Oncol. 2021 Aug 2;11:715673. doi: 10.3389/fonc.2021.715673. eCollection 2021. Front Oncol. 2021. PMID: 34408983 Free PMC article.
-
Recent progress in studies of photocages.Smart Mol. 2023 Mar 20;1(1):e20220003. doi: 10.1002/smo.20220003. eCollection 2023 Jun. Smart Mol. 2023. PMID: 40625648 Free PMC article. Review.
-
N-Confused Porphyrin Immobilized on Solid Supports: Synthesis and Metal Ions Sensing Efficacy.Molecules. 2018 Apr 10;23(4):867. doi: 10.3390/molecules23040867. Molecules. 2018. PMID: 29642601 Free PMC article.
-
α- and β-Substituted Metal-Free Phthalocyanines: Synthesis, Photophysical and Electrochemical Properties.Molecules. 2020 Jan 16;25(2):363. doi: 10.3390/molecules25020363. Molecules. 2020. PMID: 31963102 Free PMC article.
-
β-Formyl- and β-Vinylporphyrins: Magic Building Blocks for Novel Porphyrin Derivatives.Molecules. 2017 Jul 29;22(8):1269. doi: 10.3390/molecules22081269. Molecules. 2017. PMID: 28758915 Free PMC article. Review.
References
-
- Tada Y., Shikishima Y., Takaishi Y., Shibata H., Higuti T., Honda G., Ito M., Takeda Y., Kodzhimatov O.K., Ashurmetov O., et al. Coumarins and γ-pyrone derivatives from Prangos pabularia: Antibacterial activity and inhibition of cytokine release. Phytochemistry. 2002;59:649–654. doi: 10.1016/S0031-9422(02)00023-7. - DOI - PubMed
-
- Matos M.J., Vazquez-Rodriguez S., Santana L., Uriarte E., Fuentes-Edfuf C., Santos Y., Muñoz-Crego A. Synthesis and structure-activity relationships of novel amino/nitro substituted 3-arylcoumarins as antibacterial agents. Molecules. 2013;18:1394–1404. doi: 10.3390/molecules18021394. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

