Coumarin-Tetrapyrrolic Macrocycle Conjugates: Synthesis and Applications
- PMID: 28617340
- PMCID: PMC6152750
- DOI: 10.3390/molecules22060994
Coumarin-Tetrapyrrolic Macrocycle Conjugates: Synthesis and Applications
Abstract
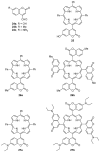
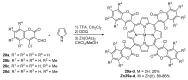
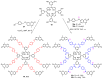
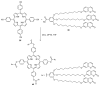
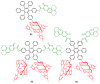
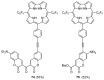
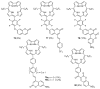
This review covers the synthesis of coumarin-porphyrin, coumarin-phthalocyanine and coumarin-corrole conjugates and their potential applications. While coumarin-phthalocyanine conjugates were obtained almost exclusively by tetramerization of coumarin-functionalized phthalonitriles, coumarin-porphyrin and coumarin-corrole conjugates were prepared by complementary approaches: (a) direct synthesis of the tetrapyrrolic macrocycle using formylcoumarins and pyrrole or (b) by functionalization of the tetrapyrrolic macrocycle. In the last approach a range of reaction types were used, namely 1,3-dipolar cycloadditions, hetero-Diels-Alder, Sonogashira, alkylation or acylation reactions. This is clearly a more versatile approach, leading to a larger diversity of conjugates and allowing the access to conjugates bearing one to up to 16 coumarin units.
Keywords: corroles; coumarins; phthalocyanines; porphyrins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




















References
-
- Tada Y., Shikishima Y., Takaishi Y., Shibata H., Higuti T., Honda G., Ito M., Takeda Y., Kodzhimatov O.K., Ashurmetov O., et al. Coumarins and γ-pyrone derivatives from Prangos pabularia: Antibacterial activity and inhibition of cytokine release. Phytochemistry. 2002;59:649–654. doi: 10.1016/S0031-9422(02)00023-7. - DOI - PubMed
-
- Matos M.J., Vazquez-Rodriguez S., Santana L., Uriarte E., Fuentes-Edfuf C., Santos Y., Muñoz-Crego A. Synthesis and structure-activity relationships of novel amino/nitro substituted 3-arylcoumarins as antibacterial agents. Molecules. 2013;18:1394–1404. doi: 10.3390/molecules18021394. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

