Antibodies Against Specific MUC16 Glycosylation Sites Inhibit Ovarian Cancer Growth
- PMID: 28617578
- PMCID: PMC5603915
- DOI: 10.1021/acschembio.7b00305
Antibodies Against Specific MUC16 Glycosylation Sites Inhibit Ovarian Cancer Growth
Abstract
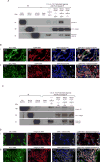
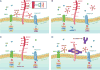
Expression of the retained C-terminal extracellular portion of the ovarian cancer glycoprotein MUC16 induces transformation and tumor growth. However, the mechanisms of MUC16 oncogenesis related to glycosylation are not clearly defined. We establish that MUC16 oncogenic effects are mediated through MGAT5-dependent N-glycosylation of two specific asparagine sites within its 58 amino acid ectodomain. Oncogenic signaling from the C-terminal portion of MUC16 requires the presence of Galectin-3 and growth factor receptors colocalized on lipid rafts. These effects are blocked upon loss of either Galectin-3 expression or activity MGAT5. Using synthetic MUC16 glycopeptides, we developed novel N-glycosylation site directed monoclonal antibodies that block Galectin-3-mediated MUC16 interactions with cell surface signaling molecules. These antibodies inhibit invasion of ovarian cancer cells, directly blocking the in vivo growth of MUC16-bearing ovarian cancer xenografts, elucidating new therapeutic modalities.
Conflict of interest statement
Memorial Sloan Kettering Cancer Center filed a patent application for MUC16 glycosylated ectodomain monoclonal antibodies on March 17, 2015, and the outcome is pending.
Figures




References
-
- Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer. 2002;98:737–40. - PubMed
-
- O’Brien TJ, et al. The CA 125 gene: an extracellular superstructure dominated by repeat sequences. Tumour Biol. 2001;22:348–66. - PubMed
-
- Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

