Identification of Padi2 as a novel angiogenesis-regulating gene by genome association studies in mice
- PMID: 28617813
- PMCID: PMC5491319
- DOI: 10.1371/journal.pgen.1006848
Identification of Padi2 as a novel angiogenesis-regulating gene by genome association studies in mice
Abstract
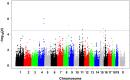
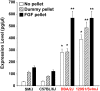
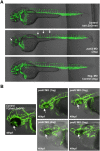
Recent findings indicate that growth factor-driven angiogenesis is markedly influenced by genetic variation. This variation in angiogenic responsiveness may alter the susceptibility to a number of angiogenesis-dependent diseases. Here, we utilized the genetic diversity available in common inbred mouse strains to identify the loci and candidate genes responsible for differences in angiogenic response. The corneal micropocket neovascularization assay was performed on 42 different inbred mouse strains using basic fibroblast growth factor (bFGF) pellets. We performed a genome-wide association study utilizing efficient mixed-model association (EMMA) mapping using the induced vessel area from all strains. Our analysis yielded five loci with genome-wide significance on chromosomes 4, 8, 11, 15 and 16. We further refined the mapping on chromosome 4 within a haplotype block containing multiple candidate genes. These genes were evaluated by expression analysis in corneas of various inbred strains and in vitro functional assays in human microvascular endothelial cells (HMVECs). Of these, we found the expression of peptidyl arginine deiminase type II (Padi2), known to be involved in metabolic pathways, to have a strong correlation with a haplotype shared by multiple high angiogenic strains. In addition, inhibition of Padi2 demonstrated a dosage-dependent effect in HMVECs. To investigate its role in vivo, we knocked down Padi2 in transgenic kdrl:zsGreen zebrafish embryos using morpholinos. These embryos had disrupted vessel formation compared to control siblings. The impaired vascular pattern was partially rescued by human PADI2 mRNA, providing evidence for the specificity of the morphant phenotype. Taken together, our study is the first to indicate the potential role of Padi2 as an angiogenesis-regulating gene. The characterization of Padi2 and other genes in associated pathways may provide new understanding of angiogenesis regulation and novel targets for diagnosis and treatment of a wide variety of angiogenesis-dependent diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Identification of Basp1 as a novel angiogenesis-regulating gene by multi-model system studies.FASEB J. 2021 May;35(5):e21404. doi: 10.1096/fj.202001936RRR. FASEB J. 2021. PMID: 33899275 Free PMC article.
-
Genetic loci that control vascular endothelial growth factor-induced angiogenesis.FASEB J. 2003 Nov;17(14):2112-4. doi: 10.1096/fj.03-0246fje. Epub 2003 Sep 4. FASEB J. 2003. PMID: 12958152
-
Peptidyl arginine deiminase 2 (Padi2) is expressed in Sertoli cells in a specific manner and regulated by SOX9 during testicular development.Sci Rep. 2018 Sep 5;8(1):13263. doi: 10.1038/s41598-018-31376-8. Sci Rep. 2018. PMID: 30185873 Free PMC article.
-
Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review.
-
Common polymorphisms in angiogenesis.Cold Spring Harb Perspect Med. 2012 Nov 1;2(11):a006510. doi: 10.1101/cshperspect.a006510. Cold Spring Harb Perspect Med. 2012. PMID: 23125197 Free PMC article. Review.
Cited by
-
Identification of Basp1 as a novel angiogenesis-regulating gene by multi-model system studies.FASEB J. 2021 May;35(5):e21404. doi: 10.1096/fj.202001936RRR. FASEB J. 2021. PMID: 33899275 Free PMC article.
-
A novel histone deacetylase inhibitor W2A-16 improves the barrier integrity in brain vascular endothelial cells.Front Cell Neurosci. 2024 Jul 19;18:1368018. doi: 10.3389/fncel.2024.1368018. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39100897 Free PMC article.
-
Angiogenic responses in a 3D micro-engineered environment of primary endothelial cells and pericytes.Angiogenesis. 2021 Feb;24(1):111-127. doi: 10.1007/s10456-020-09746-6. Epub 2020 Sep 21. Angiogenesis. 2021. PMID: 32955682
-
Applicability of Precision Medicine Approaches to Managing Hypertension in Rural Populations.J Pers Med. 2018 Apr 30;8(2):16. doi: 10.3390/jpm8020016. J Pers Med. 2018. PMID: 29710874 Free PMC article.
-
Role of the PADI family in inflammatory autoimmune diseases and cancers: A systematic review.Front Immunol. 2023 Mar 20;14:1115794. doi: 10.3389/fimmu.2023.1115794. eCollection 2023. Front Immunol. 2023. PMID: 37020554 Free PMC article.
References
-
- Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, Zhao C, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006; 314: 989–992. doi: 10.1126/science.1133807 - DOI - PubMed
-
- Bhanoori M, Arvind Babu K, Pavankumar Reddy NG, Lakshmi Rao K, Zondervan K, Deenadayal M, et al. The vascular endothelial growth factor (VEGF) +405G>C 5′-untranslated region polymorphism and increased risk of endometriosis in South Indian women: A case control study. Hum Reprod. 2005; 20: 1844–1849. doi: 10.1093/humrep/deh852 - DOI - PubMed
-
- Galan A, Ferlin A, Caretti L, Buson G, Sato G, Frigo AC, et al. Association of age-related macular degeneration with polymorphisms in vascular endothelial growth factor and its receptor. Ophthalmology. 2010; 117: 1769–1774. doi: 10.1016/j.ophtha.2010.01.030 - DOI - PubMed
-
- Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, et al. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. Exs. 1997; 79:233–269. - PubMed
-
- Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006; 312:549–560. doi: 10.1016/j.yexcr.2005.11.012 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

