Molecular diversity and frequency of the diarrheagenic enteric protozoan Giardia duodenalis and Cryptosporidium spp. in a hospital setting in Northern Spain
- PMID: 28617836
- PMCID: PMC5472271
- DOI: 10.1371/journal.pone.0178575
Molecular diversity and frequency of the diarrheagenic enteric protozoan Giardia duodenalis and Cryptosporidium spp. in a hospital setting in Northern Spain
Abstract
Background: Human giardiosis and cryptosporidiosis are caused by the enteric protozoan parasites Giardia duodenalis and Cryptosporidium spp. Both pathogens are major contributors to the global burden of diarrhoeal disease, affecting primarily children and immunodebilitated individuals in resource-poor settings. Giardiosis and cryptosporidiosis also represent an important, often underestimate, public health threat in developed countries. In Spain only limited information is currently available on the epidemiology of these infections. Molecular data on the diversity, frequency, geographical distribution, and seasonality of G. duodenalis assemblages/sub-assemblages and Cryptosporidium species/sub-genotypes are particularly scarce.
Methods: A longitudinal molecular epidemiological survey was conducted between July 2015 to September 2016 in patients referred to or attended at the Hospital San Pedro (La Rioja, Northern Spain) that tested positive for G. duodenalis (N = 106) or Cryptosporidium spp. (N = 103) by direct microscopy and/or a rapid lateral flow immunochromatographic assay. G. duodenalis infections were subsequently confirmed by real-time PCR and positive isolates assessed by multi-locus sequence genotyping of the glutamate dehydrogenase and β-giardin genes of the parasite. Cryptosporidium species and sub-genotypes were investigated at the 60 kDa glycoprotein or the small subunit ribosomal RNA genes of the parasite. Sociodemographic and clinical parameters of infected patients were also gathered and analysed.
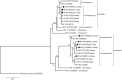
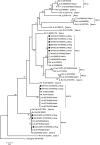
Principal findings: Out of 90 G. duodenalis-positive isolates by real-time PCR a total of 16 isolates were successfully typed. AII (44%, 7/16) was the most prevalent sub-assemblage found, followed by BIV (31%, 5/16) and BIII (19%, 3/16). A discordant genotype result AII/AIII was identified in an additional (6%, 1/16) isolate. No mixed infections A+B were detected. Similarly, a total of 81 Cryptosporidium spp. isolates were successfully typed, revealing the presence of C. hominis (81%, 66/81) and C. parvum (19%, 15/81). Obtained GP60 sequences were assigned to sub-type families Ib (73%, 59/81) within C. hominis, and IIa (7%, 6/81) and IId (2%, 2/81) within C. parvum. A marked inter-annual variation in Cryptosporidium cases was observed.
Conclusions: Human giardiasis and cryptosporidiosis are commonly identified in patients seeking medical care in Northern Spain and represent a more important health concern than initially thought. Assemblage A within G. duodenalis and sub-genotype IbA10G2 within C. hominis were the genetic variants of these parasite species more frequently found circulating in the population under study. Molecular data presented here seem to suggest that G. duodenalis and Cryptosporidium infections arise through anthroponotic rather than zoonotic transmission in this Spanish region.
Conflict of interest statement
Figures

References
-
- Checkley W, White AC, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect Dis. 2015; 15: 85–94. doi: 10.1016/S1473-3099(14)70772-8 - DOI - PMC - PubMed
-
- Halliez MC, Buret AG. Extra-intestinal and long term consequences of Giardia duodenalis infections. World J Gastroenterol. 2013; 19: 8974–8985. doi: 10.3748/wjg.v19.i47.8974 - DOI - PMC - PubMed
-
- Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg. 1999; 61: 707–713. - PubMed
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013; 382: 209–22. doi: 10.1016/S0140-6736(13)60844-2 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

