Wolbachia elevates host methyltransferase expression to block an RNA virus early during infection
- PMID: 28617844
- PMCID: PMC5472326
- DOI: 10.1371/journal.ppat.1006427
Wolbachia elevates host methyltransferase expression to block an RNA virus early during infection
Abstract
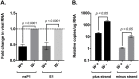
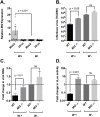
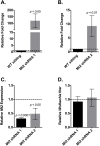
Wolbachia pipientis is an intracellular endosymbiont known to confer host resistance against RNA viruses in insects. However, the causal mechanism underlying this antiviral defense remains poorly understood. To this end, we have established a robust arthropod model system to study the tripartite interaction involving Sindbis virus and Wolbachia strain wMel within its native host, Drosophila melanogaster. By leveraging the power of Drosophila genetics and a parallel, highly tractable D. melanogaster derived JW18 cell culture system, we determined that in addition to reducing infectious virus production, Wolbachia negatively influences Sindbis virus particle infectivity. This is further accompanied by reductions in viral transcript and protein levels. Interestingly, unchanged ratio of proteins to viral RNA copies suggest that Wolbachia likely does not influence the translational efficiency of viral transcripts. Additionally, expression analyses of candidate host genes revealed D. melanogaster methyltransferase gene Mt2 as an induced host factor in the presence of Wolbachia. Further characterization of viral resistance in Wolbachia-infected flies lacking functional Mt2 revealed partial recovery of virus titer relative to wild-type, accompanied by complete restoration of viral RNA and protein levels, suggesting that Mt2 acts at the stage of viral genome replication. Finally, knockdown of Mt2 in Wolbachia uninfected JW18 cells resulted in increased virus infectivity, thus demonstrating its previously unknown role as an antiviral factor against Sindbis virus. In conclusion, our findings provide evidence supporting the role of Wolbachia-modulated host factors towards RNA virus resistance in arthropods, alongside establishing Mt2's novel antiviral function against Sindbis virus in D. melanogaster.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
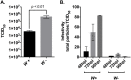

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

