Hypoxia-inducible factor-1α plays roles in Epstein-Barr virus's natural life cycle and tumorigenesis by inducing lytic infection through direct binding to the immediate-early BZLF1 gene promoter
- PMID: 28617871
- PMCID: PMC5487075
- DOI: 10.1371/journal.ppat.1006404
Hypoxia-inducible factor-1α plays roles in Epstein-Barr virus's natural life cycle and tumorigenesis by inducing lytic infection through direct binding to the immediate-early BZLF1 gene promoter
Abstract
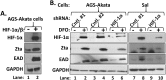
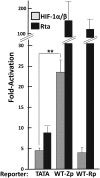
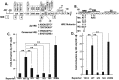
When confronted with poor oxygenation, cells adapt by activating survival signaling pathways, including the oxygen-sensitive transcriptional regulators called hypoxia-inducible factor alphas (HIF-αs). We report here that HIF-1α also regulates the life cycle of Epstein-Barr virus (EBV). Incubation of EBV-positive gastric carcinoma AGS-Akata and SNU-719 and Burkitt lymphoma Sal and KemIII cell lines with a prolyl hydroxylase inhibitor, L-mimosine or deferoxamine, or the NEDDylation inhibitor MLN4924 promoted rapid and sustained accumulation of both HIF-1α and lytic EBV antigens. ShRNA knockdown of HIF-1α significantly reduced deferoxamine-mediated lytic reactivation. HIF-1α directly bound the promoter of the EBV primary latent-lytic switch BZLF1 gene, Zp, activating transcription via a consensus hypoxia-response element (HRE) located at nt -83 through -76 relative to the transcription initiation site. HIF-1α did not activate transcription from the other EBV immediate-early gene, BRLF1. Importantly, expression of HIF-1α induced EBV lytic-gene expression in cells harboring wild-type EBV, but not in cells infected with variants containing base-pair substitution mutations within this HRE. Human oral keratinocyte (NOK) and gingival epithelial (hGET) cells induced to differentiate by incubation with either methyl cellulose or growth in organotypic culture accumulated both HIF-1α and Blimp-1α, another cellular factor implicated in lytic reactivation. HIF-1α activity also accumulated along with Blimp-1α during B-cell differentiation into plasma cells. Furthermore, most BZLF1-expressing cells observed in lymphomas induced by EBV in NSG mice with a humanized immune system were located distal to blood vessels in hypoxic regions of the tumors. Thus, we conclude that HIF-1α plays central roles in both EBV's natural life cycle and EBV-associated tumorigenesis. We propose that drugs that induce HIF-1α protein accumulation are good candidates for development of a lytic-induction therapy for treating some EBV-associated malignancies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

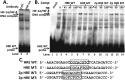
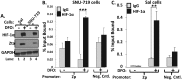

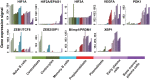


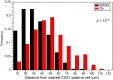
References
-
- Longnecker RM, Kieff E, Cohen JI. Epstein-Barr virus Fields Virology. 6th edition Lippincott, Williams, and Willams; 2013.
-
- Thorley-Lawson DA. EBV Persistence—Introducing the Virus. Curr Top Microbiol Immunol. 2015;390: 151–209. doi: 10.1007/978-3-319-22822-8_8 - DOI - PMC - PubMed
-
- Kang M-S, Kieff E. Epstein-Barr virus latent genes. Exp Mol Med. 2015;47: e131 doi: 10.1038/emm.2014.84 - DOI - PMC - PubMed
-
- Kutok JL, Wang F. Spectrum of Epstein-Barr virus-associated diseases. Annu Rev Pathol. 2006;1: 375–404. doi: 10.1146/annurev.pathol.1.110304.100209 - DOI - PubMed
-
- Rickinson AB. Co-infections, inflammation and oncogenesis: future directions for EBV research. Semin Cancer Biol. 2014;26: 99–115. doi: 10.1016/j.semcancer.2014.04.004 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

