Optical coherence tomography angiography-based capillary velocimetry
- PMID: 28617921
- PMCID: PMC5472241
- DOI: 10.1117/1.JBO.22.6.066008
Optical coherence tomography angiography-based capillary velocimetry
Abstract
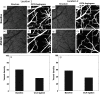
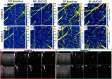
Challenge persists in the field of optical coherence tomography (OCT) when it is required to quantify capillary blood flow within tissue beds in vivo. We propose a useful approach to statistically estimate the mean capillary flow velocity using a model-based statistical method of eigendecomposition (ED) analysis of the complex OCT signals obtained with the OCT angiography (OCTA) scanning protocol. ED-based analysis is achieved by the covariance matrix of the ensemble complex OCT signals, upon which the eigenvalues and eigenvectors that represent the subsets of the signal makeup are calculated. From this analysis, the signals due to moving particles can be isolated by employing an adaptive regression filter to remove the eigencomponents that represent static tissue signals. The mean frequency (MF) of moving particles can be estimated by the first lag-one autocorrelation of the corresponding eigenvectors. Three important parameters are introduced, including the blood flow signal power representing the presence of blood flow (i.e., OCTA signals), the MF indicating the mean velocity of blood flow, and the frequency bandwidth describing the temporal flow heterogeneity within a scanned tissue volume. The proposed approach is tested using scattering phantoms, in which microfluidic channels are used to simulate the functional capillary vessels that are perfused with the scattering intralipid solution. The results indicate a linear relationship between the MF and mean flow velocity. In vivo animal experiments are also conducted by imaging mouse brain with distal middle cerebral artery ligation to test the capability of the method to image the changes in capillary flows in response to an ischemic insult, demonstrating the practical usefulness of the proposed method for providing important quantifiable information about capillary tissue beds in the investigations of neurological conditions in vivo.
Figures






References
-
- Fercher A., et al. , “Optical coherence tomography-principles and applications,” Rep. Prog. Phys. 66(2), 239–303 (2003).RPPHAG10.1088/0034-4885/66/2/204 - DOI
-
- Tomlins P. H., Wang R. K., “Theory, developments and applications of optical coherence tomography,” J. Phys. D Appl. Phys. 38(15), 2519 (2005).10.1088/0022-3727/38/15/002 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

