Ambient and Dosed Exposure to Quaternary Ammonium Disinfectants Causes Neural Tube Defects in Rodents
- PMID: 28618200
- PMCID: PMC5905424
- DOI: 10.1002/bdr2.1064
Ambient and Dosed Exposure to Quaternary Ammonium Disinfectants Causes Neural Tube Defects in Rodents
Abstract
Background: Quaternary ammonium compounds are a large class of chemicals used for their antimicrobial and antistatic properties. Two common quaternary ammonium compounds, alkyldimethylbenzyl ammonium chloride (ADBAC) and didecyldimethyl ammonium chloride (DDAC), are combined in common cleaners and disinfectants. Introduction of a cleaner containing ADBAC+DDAC in the vivarium caused neural tube defects (NTDs) in mice and rats.
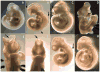
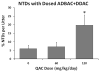
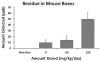
Methods: To further evaluate this finding, male and female mice were dosed in the feed at 60 or 120 mg/kg/day, or by oral gavage at 7.5, 15, or 30 mg/kg ADBAC+DDAC. Mice also received ambient exposure to ADBAC+DDAC from the disinfectant used in the mouse room. Embryos were evaluated on gestational day 10 for NTDs, and fetuses were evaluated on gestational day 18 for gross and skeletal malformations.
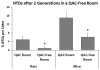
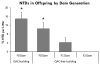
Results: We found increased NTDs with exposure to ADBAC+DDAC in both rats and mice. The NTDs persisted for two generations after cessation of exposure. Notably, male exposure alone was sufficient to cause NTDs. Equally significant, ambient exposure from disinfectant use in the vivarium, influenced the levels of NTDs to a greater extent than oral dosing. No gross or significant axial skeletal malformations were observed in late gestation fetuses. Placental abnormalities and late gestation fetal deaths were increased at 120 mg/kg/day, which might explain the lack of malformations observed in late gestation fetuses.
Conclusion: These results demonstrate that ADBAC+DDAC in combination are teratogenic to rodents. Given the increased use of these disinfectants, further evaluation of their safety in humans and their contribution to health and disease is essential. Birth Defects Research 109:1166-1178, 2017. © 2017 The Authors. Birth Defects Research Published by Wiley Periodicals, Inc.
Keywords: QAC disinfectants; QACs; abnormal development; environmental contaminants; neural tube defects; teratogenesis.
© 2017 The Authors. Birth Defects Research Published by Wiley Periodicals, Inc.
Figures






Comment in
-
Response to Hostetler.Birth Defects Res. 2018 Apr 3;110(6):545-546. doi: 10.1002/bdr2.1193. Epub 2018 Jan 16. Birth Defects Res. 2018. PMID: 29341440 No abstract available.
-
Comments on "Ambient and Dosed Exposure to Quaternary Ammonium Disinfectants Causes Neural Tube Defects in Rodents".Birth Defects Res. 2018 Apr 3;110(6):543-544. doi: 10.1002/bdr2.1194. Epub 2018 Feb 1. Birth Defects Res. 2018. PMID: 29388359 No abstract available.
References
-
- Bernstein JA, Staudder T, Bernstein DI, et al. A combined respiratory and cutaneous hypersensitivity syndrome induced by work exposure to quaternary amines. J Allergy Clin Immunol. 1994;94:257–259. - PubMed
-
- Boyle CE, Boulet S, Schieve LA, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127:1034–1042. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

