Non-inferiority versus superiority drug claims: the (not so) subtle distinction
- PMID: 28619049
- PMCID: PMC5472861
- DOI: 10.1186/s13063-017-2024-2
Non-inferiority versus superiority drug claims: the (not so) subtle distinction
Abstract
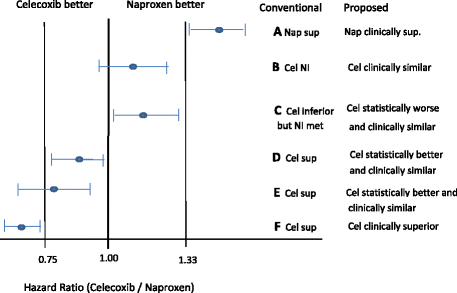
Background: Current regulatory guidance and practice of non-inferiority trials are asymmetric in favor of the test treatment (Test) over the reference treatment (Control). These trials are designed to compare the relative efficacy of Test to Control by reference to a clinically important margin, M.
Main text: Non-inferiority trials allow for the conclusion of: (a) non-inferiority of Test to Control if Test is slightly worse than Control but by no more than M; and (b) superiority if Test is slightly better than Control even if it is by less than M. From Control's perspective, (b) should lead to a conclusion of non-inferiority of Control to Test. The logical interpretation ought to be that, while Test is statistically better, it is not clinically superior to Control (since Control should be able to claim non-inferiority to Test). This article makes a distinction between statistical and clinical significance, providing for symmetry in the interpretation of results. Statistical superiority and clinical superiority are achieved, respectively, when the null and the non-inferiority margins are exceeded. We discuss a similar modification to placebo-controlled trials.
Conclusion: Rules for interpretation should not favor one treatment over another. Claims of statistical or clinical superiority should depend on whether or not the null margin or the clinically relevant margin is exceeded.
Keywords: Confidence interval; Margin; Non-inferiority; Superiority; Trial design.
Figures
Similar articles
-
Superiority and non-inferiority: two sides of the same coin?Trials. 2018 Sep 17;19(1):499. doi: 10.1186/s13063-018-2885-z. Trials. 2018. PMID: 30223881 Free PMC article.
-
Optimal Use of the Non-Inferiority Trial Design.Pharmaceut Med. 2020 Jun;34(3):159-165. doi: 10.1007/s40290-020-00334-z. Pharmaceut Med. 2020. PMID: 32277352 Review.
-
Testing superiority and non-inferiority hypotheses in active controlled clinical trials.Biom J. 2005 Feb;47(1):62-74; discussion 99-107. doi: 10.1002/bimj.200410089. Biom J. 2005. PMID: 16395997
-
A unifying approach to non-inferiority, equivalence and superiority tests via multiple decision processes.Pharm Stat. 2007 Jul-Sep;6(3):193-203. doi: 10.1002/pst.305. Pharm Stat. 2007. PMID: 17879327
-
A review of UK publicly funded non-inferiority trials: is the design more inferior than it should be?Trials. 2024 Dec 4;25(1):809. doi: 10.1186/s13063-024-08651-3. Trials. 2024. PMID: 39627838 Free PMC article. Review.
Cited by
-
Non-inferiority and clinical superiority of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors: Systematic analysis of cardiorenal outcome trials in type 2 diabetes.Diabetes Obes Metab. 2022 Aug;24(8):1598-1606. doi: 10.1111/dom.14735. Epub 2022 May 29. Diabetes Obes Metab. 2022. PMID: 35491523 Free PMC article.
-
Superiority and non-inferiority: two sides of the same coin?Trials. 2018 Sep 17;19(1):499. doi: 10.1186/s13063-018-2885-z. Trials. 2018. PMID: 30223881 Free PMC article.
-
Non-inferiority trials.Perspect Clin Res. 2022 Jan-Mar;13(1):54-57. doi: 10.4103/picr.picr_245_21. Epub 2022 Jan 6. Perspect Clin Res. 2022. PMID: 35198430 Free PMC article.
-
Optimal contrast analysis with heterogeneous variances and budget concerns.PLoS One. 2019 Mar 26;14(3):e0214391. doi: 10.1371/journal.pone.0214391. eCollection 2019. PLoS One. 2019. PMID: 30913244 Free PMC article.
-
Non-inferiority trials in clinical ophthalmology: a systematic review.Eye (Lond). 2025 Aug;39(11):2151-2158. doi: 10.1038/s41433-025-03819-w. Epub 2025 May 1. Eye (Lond). 2025. PMID: 40312555
References
-
- FDA Guidance for Industry. Non-inferiority clinical trials to establish effectiveness. 2016. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformati.... Accessed 12 June 2017.
-
- The European Agency for Evaluation of Medical Products. Points to consider on switching between superiority and non-inferiority. 2000. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... Accessed 12 June 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources