Reducing the rate and duration of Re-ADMISsions among patients with unipolar disorder and bipolar disorder using smartphone-based monitoring and treatment - the RADMIS trials: study protocol for two randomized controlled trials
- PMID: 28619114
- PMCID: PMC5472886
- DOI: 10.1186/s13063-017-2015-3
Reducing the rate and duration of Re-ADMISsions among patients with unipolar disorder and bipolar disorder using smartphone-based monitoring and treatment - the RADMIS trials: study protocol for two randomized controlled trials
Abstract
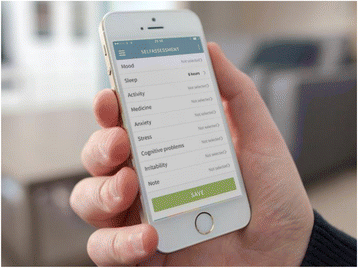
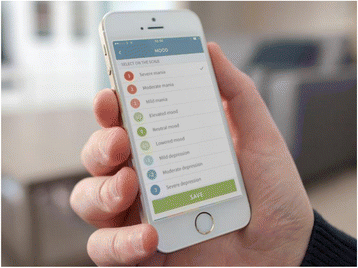
Background: Unipolar and bipolar disorder combined account for nearly half of all morbidity and mortality due to mental and substance use disorders, and burden society with the highest health care costs of all psychiatric and neurological disorders. Among these, costs due to psychiatric hospitalization are a major burden. Smartphones comprise an innovative and unique platform for the monitoring and treatment of depression and mania. No prior trial has investigated whether the use of a smartphone-based system can prevent re-admission among patients discharged from hospital. The present RADMIS trials aim to investigate whether using a smartphone-based monitoring and treatment system, including an integrated clinical feedback loop, reduces the rate and duration of re-admissions more than standard treatment in unipolar disorder and bipolar disorder.
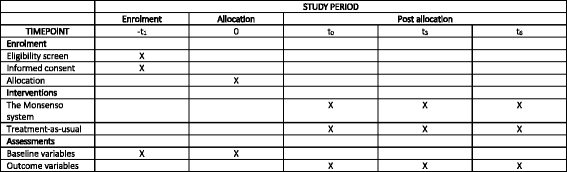
Methods: The RADMIS trials use a randomized controlled, single-blind, parallel-group design. Patients with unipolar disorder and patients with bipolar disorder are invited to participate in each trial when discharged from psychiatric hospitals in The Capital Region of Denmark following an affective episode and randomized to either (1) a smartphone-based monitoring system including (a) an integrated feedback loop between patients and clinicians and (b) context-aware cognitive behavioral therapy (CBT) modules (intervention group) or (2) standard treatment (control group) for a 6-month trial period. The trial started in May 2017. The outcomes are (1) number and duration of re-admissions (primary), (2) severity of depressive and manic (only for patients with bipolar disorder) symptoms; psychosocial functioning; number of affective episodes (secondary), and (3) perceived stress, quality of life, self-rated depressive symptoms, self-rated manic symptoms (only for patients with bipolar disorder), recovery, empowerment, adherence to medication, wellbeing, ruminations, worrying, and satisfaction (tertiary). A total of 400 patients (200 patients with unipolar disorder and 200 patients with bipolar disorder) will be included in the RADMIS trials.
Discussion: If the smartphone-based monitoring system proves effective in reducing the rate and duration of re-admissions, there will be basis for using a system of this kind in the treatment of unipolar and bipolar disorder in general and on a larger scale.
Trial registration: ClinicalTrials.gov, ID: NCT03033420 . Registered 13 January 2017. Ethical approval has been obtained.
Figures

Similar articles
-
Daily electronic monitoring of subjective and objective measures of illness activity in bipolar disorder using smartphones--the MONARCA II trial protocol: a randomized controlled single-blind parallel-group trial.BMC Psychiatry. 2014 Nov 25;14:309. doi: 10.1186/s12888-014-0309-5. BMC Psychiatry. 2014. PMID: 25420431 Free PMC article. Clinical Trial.
-
The effect of smartphone-based monitoring and treatment including clinical feedback versus smartphone-based monitoring without clinical feedback in bipolar disorder: the SmartBipolar trial-a study protocol for a randomized controlled parallel-group trial.Trials. 2023 Sep 12;24(1):583. doi: 10.1186/s13063-023-07625-1. Trials. 2023. PMID: 37700334 Free PMC article.
-
Reducing the rate of psychiatric re-admissions in bipolar disorder using smartphones-The RADMIS trial.Acta Psychiatr Scand. 2021 May;143(5):453-465. doi: 10.1111/acps.13274. Epub 2021 Jan 26. Acta Psychiatr Scand. 2021. PMID: 33354769 Clinical Trial.
-
ECNP consensus meeting. Bipolar depression. Nice, March 2007.Eur Neuropsychopharmacol. 2008 Jul;18(7):535-49. doi: 10.1016/j.euroneuro.2008.03.003. Epub 2008 May 23. Eur Neuropsychopharmacol. 2008. PMID: 18501566 Review.
-
Electronic monitoring in bipolar disorder.Dan Med J. 2018 Mar;65(3):B5460. Dan Med J. 2018. PMID: 29510813 Review.
Cited by
-
Circadian reinforcement therapy in combination with electronic self-monitoring to facilitate a safe post-discharge period of patients with depression by stabilizing sleep: protocol of a randomized controlled trial.BMC Psychiatry. 2019 Apr 25;19(1):124. doi: 10.1186/s12888-019-2101-z. BMC Psychiatry. 2019. PMID: 31023274 Free PMC article. Clinical Trial.
-
Smartphone-based objective monitoring in bipolar disorder: status and considerations.Int J Bipolar Disord. 2018 Jan 23;6(1):6. doi: 10.1186/s40345-017-0110-8. Int J Bipolar Disord. 2018. PMID: 29359252 Free PMC article. Review.
-
Mood and Activity Measured Using Smartphones in Unipolar Depressive Disorder.Front Psychiatry. 2021 Jul 9;12:701360. doi: 10.3389/fpsyt.2021.701360. eCollection 2021. Front Psychiatry. 2021. PMID: 34366933 Free PMC article.
-
Telemedicine as a Tool to Improve Medicine Adherence in Patients with Affective Disorders - A Systematic Literature Review.Patient Prefer Adherence. 2022 Dec 30;16:3441-3463. doi: 10.2147/PPA.S388106. eCollection 2022. Patient Prefer Adherence. 2022. PMID: 36605330 Free PMC article. Review.
-
Forecasting Mood in Bipolar Disorder From Smartphone Self-assessments: Hierarchical Bayesian Approach.JMIR Mhealth Uhealth. 2020 Apr 1;8(4):e15028. doi: 10.2196/15028. JMIR Mhealth Uhealth. 2020. PMID: 32234702 Free PMC article. Clinical Trial.
References
-
- Arbejdsgruppe under Regeringens udvalg om Psykiatri. Indsatsen for mennesker med psykiske lidelser- kapacitet, sammenhæng og strktur. Bilagsrapport 1. Afrapportering fra Arbejdsgruppemøde under Regeringens udvalg om Psykiatri. 2013.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

