Breast Cancer Neoantigens Can Induce CD8+ T-Cell Responses and Antitumor Immunity
- PMID: 28619968
- PMCID: PMC5647648
- DOI: 10.1158/2326-6066.CIR-16-0264
Breast Cancer Neoantigens Can Induce CD8+ T-Cell Responses and Antitumor Immunity
Abstract
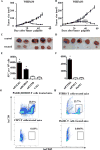
Next-generation sequencing technologies have provided insights into the biology and mutational landscape of cancer. Here, we evaluate the relevance of cancer neoantigens in human breast cancers. Using patient-derived xenografts from three patients with advanced breast cancer (xenografts were designated as WHIM30, WHIM35, and WHIM37), we sequenced exomes of tumor and patient-matched normal cells. We identified 2,091 (WHIM30), 354 (WHIM35), and 235 (WHIM37) nonsynonymous somatic mutations. A computational analysis identified and prioritized HLA class I-restricted candidate neoantigens expressed in the dominant tumor clone. Each candidate neoantigen was evaluated using peptide-binding assays, T-cell cultures that measure the ability of CD8+ T cells to recognize candidate neoantigens, and preclinical models in which we measured antitumor immunity. Our results demonstrate that breast cancer neoantigens can be recognized by the immune system, and that human CD8+ T cells enriched for prioritized breast cancer neoantigens were able to protect mice from tumor challenge with autologous patient-derived xenografts. We conclude that next-generation sequencing and epitope-prediction strategies can identify and prioritize candidate neoantigens for immune targeting in breast cancer. Cancer Immunol Res; 5(7); 516-23. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures
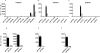
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

