Role of the anterior cingulate cortex in the retrieval of novel object recognition memory after a long delay
- PMID: 28620078
- PMCID: PMC5473111
- DOI: 10.1101/lm.044784.116
Role of the anterior cingulate cortex in the retrieval of novel object recognition memory after a long delay
Abstract
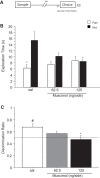
Previous in vivo electrophysiological studies suggest that the anterior cingulate cortex (ACgx) is an important substrate of novel object recognition (NOR) memory. However, intervention studies are needed to confirm this conclusion and permanent lesion studies cannot distinguish effects on encoding and retrieval. The interval between encoding and retrieval tests may also be a critical determinant of the role of the ACgx. The current series of experiments used micro-infusion of the GABAA receptor agonist, muscimol, into ACgx to reversibly inactivate the area and distinguish its role in encoding and retrieval. ACgx infusions of muscimol, before encoding did not alter NOR assessed after a delay of 20 min or 24 h. However, when infused into the ACgx before retrieval muscimol impaired NOR assessed after a delay of 24 h, but not after a 20-min retention test. Together these findings suggest that the ACgx plays a time-dependent role in the retrieval, but not the encoding, of NOR memory, neuronal activation being required for the retrieval of remote (24 h old), but not recent (20 min old) visual memory.
© 2017 Pezze et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
-
- Akirav I, Maroun M. 2006. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex 16: 1759–1765. - PubMed
-
- Banks PJ, Bashir ZI, Brown MW. 2012. Recognition memory and synaptic plasticity in the perirhinal and prefrontal cortices. Hippocampus 22: 2012–2031. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
