Ethyl Vanillin Activates TRPA1
- PMID: 28620120
- PMCID: PMC5539581
- DOI: 10.1124/jpet.116.239384
Ethyl Vanillin Activates TRPA1
Abstract
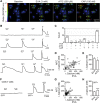
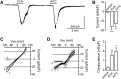
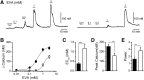
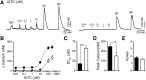
The nonselective cation channel transient receptor potential ankryn subtype family 1 (TRPA1) is expressed in neurons of dorsal root ganglia and trigeminal ganglia and also in vagal afferent neurons that innervate the lungs and gastrointestinal tract. Many TRPA1 agonists are reactive electrophilic compounds that form covalent adducts with TRPA1. Allyl isothiocyanate (AITC), the common agonist used to identify TRPA1, contains an electrophilic group that covalently binds with cysteine residues of TRPA1 and confers a structural change on the channel. There is scientific motivation to identify additional compounds that can activate TRPA1 with different mechanisms of channel gating. We provide evidence that ethyl vanillin (EVA) is a TRPA1 agonist. Using fluorescent calcium imaging and whole-cell patch-clamp electrophysiology on dissociated rat vagal afferent neurons and TRPA1-transfected COS-7 cells, we discovered that EVA activates cells also activated by AITC. Both agonists display similar current profiles and conductances. Pretreatment with A967079, a selective TRPA1 antagonist, blocks the EVA response as well as the AITC response. Furthermore, EVA does not activate vagal afferent neurons from TRPA1 knockout mice, showing selectivity for TRPA1 in this tissue. Interestingly, EVA appears to be pharmacologically different from AITC as a TRPA1 agonist. When AITC is applied before EVA, the EVA response is occluded. However, they both require intracellular oxidation to activate TRPA1. These findings suggest that EVA activates TRPA1 but via a distinct mechanism that may provide greater ease for study in native systems compared with AITC and may shed light on differential modes of TRPA1 gating by ligand types.
Copyright © 2017 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



Similar articles
-
Nitro-oleic acid desensitizes TRPA1 and TRPV1 agonist responses in adult rat DRG neurons.Exp Neurol. 2014 Jan;251:12-21. doi: 10.1016/j.expneurol.2013.10.020. Epub 2013 Nov 8. Exp Neurol. 2014. PMID: 24212047 Free PMC article.
-
Activation characteristics of transient receptor potential ankyrin 1 and its role in nociception.Am J Physiol Cell Physiol. 2011 Sep;301(3):C587-600. doi: 10.1152/ajpcell.00465.2010. Epub 2011 Jun 8. Am J Physiol Cell Physiol. 2011. PMID: 21653898 Free PMC article.
-
Activation of transient receptor potential ankyrin 1 by eugenol.Neuroscience. 2014 Mar 7;261:153-60. doi: 10.1016/j.neuroscience.2013.12.047. Epub 2013 Dec 30. Neuroscience. 2014. PMID: 24384226
-
Nitro-oleic acid inhibits firing and activates TRPV1- and TRPA1-mediated inward currents in dorsal root ganglion neurons from adult male rats.J Pharmacol Exp Ther. 2010 Jun;333(3):883-95. doi: 10.1124/jpet.109.163154. Epub 2010 Mar 19. J Pharmacol Exp Ther. 2010. PMID: 20304940 Free PMC article.
-
Transient receptor potential A1 mediates an osmotically activated ion channel.Eur J Neurosci. 2008 Feb;27(3):605-11. doi: 10.1111/j.1460-9568.2008.06030.x. Eur J Neurosci. 2008. PMID: 18279313
Cited by
-
Electronic Cigarettes: Their Constituents and Potential Links to Asthma.Curr Allergy Asthma Rep. 2017 Oct 5;17(11):79. doi: 10.1007/s11882-017-0747-5. Curr Allergy Asthma Rep. 2017. PMID: 28983782 Free PMC article. Review.
-
Ice flavours and non-menthol synthetic cooling agents in e-cigarette products: a review.Tob Control. 2023 Nov;32(6):769-777. doi: 10.1136/tobaccocontrol-2021-057073. Epub 2022 Apr 28. Tob Control. 2023. PMID: 35483721 Free PMC article. Review.
-
Targeting Chemosensory Ion Channels in Peripheral Swallowing-Related Regions for the Management of Oropharyngeal Dysphagia.Int J Mol Sci. 2020 Aug 27;21(17):6214. doi: 10.3390/ijms21176214. Int J Mol Sci. 2020. PMID: 32867366 Free PMC article. Review.
-
Harnessing migraines for neural regeneration.Neural Regen Res. 2018 Apr;13(4):609-615. doi: 10.4103/1673-5374.230275. Neural Regen Res. 2018. PMID: 29722303 Free PMC article. Review.
-
TRPV1 enhances cholecystokinin signaling in primary vagal afferent neurons and mediates the central effects on spontaneous glutamate release in the NTS.Am J Physiol Cell Physiol. 2024 Jan 1;326(1):C112-C124. doi: 10.1152/ajpcell.00409.2023. Epub 2023 Dec 4. Am J Physiol Cell Physiol. 2024. PMID: 38047304 Free PMC article.
References
-
- Alvarez-Berdugo D, Rofes L, Farré R, Casamitjana JF, Enrique A, Chamizo J, Padrón A, Navarro X, Clavé P. (2016) Localization and expression of TRPV1 and TRPA1 in the human oropharynx and larynx. Neurogastroenterol Motil 28:91–100. - PubMed
-
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857. - PubMed
-
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–1282. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

