Formation of Biphasic Hydroxylapatite-Beta Magnesium Tricalcium Phosphate in Heat Treated Salmonid Vertebrae
- PMID: 28620190
- PMCID: PMC5472584
- DOI: 10.1038/s41598-017-03737-2
Formation of Biphasic Hydroxylapatite-Beta Magnesium Tricalcium Phosphate in Heat Treated Salmonid Vertebrae
Abstract
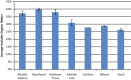
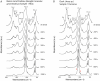
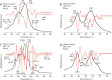
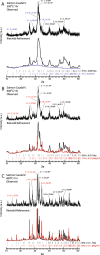
Ichthyoarchaeological evidence is uncommon at ancient hunter-gatherer sites from various regions and timeframes. This research contributes to the development of microarchaeological techniques useful for identifying fishing economies in situations where classifiable bones are unavailable. Specifically, traces of heat altered bone mineral in domestic hearths are expected to provide markers for discarded fish remains. We used a series of laboratory incineration experiments to characterize the mineralogy of burned salmonid vertebrae. Fourier transform infrared spectroscopy and x-ray diffraction distinguished the formation of beta magnesium tricalcium phosphate (βMgTCP) at temperatures as low as 600 °C. Bones from a sample of game mammals and birds did not form this phase at temperatures below 1,000 °C. We propose that this neoformed mineral can serve as a proxy for hunter-gatherer salmonid fishing when typical ichthyoarchaeological evidence is absent. Using Fourier transform infrared spectroscopy, it will be possible to rapidly and inexpensively determine the presence of βMgTCP in fragmentary burned bone remains associated with combustion features. The occurrence of βMgTCP in archaeological hearth features will offer a new means of further evaluating the temporal, geographic, and cultural scope of salmonid harvesting. We also acknowledge the value of biphasic hydroxylapatite-βMgTCP recovered from Atlantic salmon vertebrae as a bioceramic.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Msangi, S. et al. Fish to 2030: prospects for fisheries and aquaculture. http://www.fao.org/docrep/019/i3640e/i3640e.pdf (World Bank, 2013).
-
- Food and Agriculture Organization of the United Nations, Species Fact Sheet, Atlantic Salmon. http://www.fao.org/fishery/species/2929/en (2016).
-
- Katz, J. Salmon farming in Chile. In Technology, Adaptation, and Exports - How Some Developing Countries Got it Right (eds Chandra, V. & Kolavalli, S.) (World Bank, 2006).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

