Novel bioactive glass based injectable bone cement with improved osteoinductivity and its in vivo evaluation
- PMID: 28620229
- PMCID: PMC5472605
- DOI: 10.1038/s41598-017-03207-9
Novel bioactive glass based injectable bone cement with improved osteoinductivity and its in vivo evaluation
Abstract
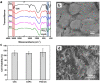
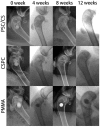
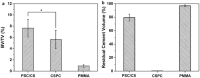
Recently, more and more attention has been paid to the development of a new generation of injectable bone cements that are bioactive, biodegradable and are able to have appropriate mechanical properties for treatment of vertebral compression fractures (VCFs). In this study, a novel PSC/CS composite cement with high content of PSC (a phytic acid-derived bioactive glass) was prepared and evaluated in both vitro and vivo. The PSC/CS cement showed excellent injectability, good resistance to disintegration, radiopacity and suitable mechanical properties. The in vitro test showed that the cement was bioactive, biocompatible and could maintain its shape sustainably, which made it possible to provide a long-term mechanical support for bone regeneration. Radiography, microcomputed tomography and histology of critical sized rabbit femoral condyle defects implanted with the cements proved the resorption and osteoinductivity of the cement. Compared with the PMMA and CSPC, there were more osteocyte and trabeculae at the Bone-Cement interface in the group PSC/CS cement. The volume of the residual bone cement suggested that PSC/CS had certain ability of degradation and the resorption rate was much lower than that of the CSPC cement. Together, the results indicated that the cement was a promising bone cement to treat the VCFs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Evaluation of injectable strontium-containing borate bioactive glass cement with enhanced osteogenic capacity in a critical-sized rabbit femoral condyle defect model.ACS Appl Mater Interfaces. 2015 Feb 4;7(4):2393-403. doi: 10.1021/am507008z. Epub 2015 Jan 23. ACS Appl Mater Interfaces. 2015. PMID: 25591177
-
An injectable pH neutral bioactive glass-based bone cement with suitable bone regeneration ability.J Orthop Translat. 2022 Sep 2;36:120-131. doi: 10.1016/j.jot.2022.05.011. eCollection 2022 Sep. J Orthop Translat. 2022. PMID: 36128442 Free PMC article.
-
Evaluation of an injectable bioactive borate glass cement to heal bone defects in a rabbit femoral condyle model.Mater Sci Eng C Mater Biol Appl. 2017 Apr 1;73:585-595. doi: 10.1016/j.msec.2016.12.101. Epub 2016 Dec 22. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 28183648
-
Comparison of Percutaneous Vertebroplasty and Balloon Kyphoplasty for the Treatment of Single Level Vertebral Compression Fractures: A Meta-analysis of the Literature.Pain Physician. 2015 May-Jun;18(3):209-22. Pain Physician. 2015. PMID: 26000665 Review.
-
Sudoku of porous, injectable calcium phosphate cements - Path to osteoinductivity.Bioact Mater. 2022 Jan 10;17:109-124. doi: 10.1016/j.bioactmat.2022.01.001. eCollection 2022 Nov. Bioact Mater. 2022. PMID: 35386461 Free PMC article. Review.
Cited by
-
Biocomposite Macrospheres Based on Strontium-Bioactive Glass for Application as Bone Fillers.ACS Mater Au. 2023 Aug 1;3(6):646-658. doi: 10.1021/acsmaterialsau.3c00048. eCollection 2023 Nov 8. ACS Mater Au. 2023. PMID: 38089665 Free PMC article.
-
Novel Osteogenic Behaviors around Hydrophilic and Radical-Free 4-META/MMA-TBB: Implications of an Osseointegrating Bone Cement.Int J Mol Sci. 2020 Mar 31;21(7):2405. doi: 10.3390/ijms21072405. Int J Mol Sci. 2020. PMID: 32244335 Free PMC article.
-
A novel bioactive glass-based root canal sealer in endodontics.J Dent Sci. 2022 Jan;17(1):217-224. doi: 10.1016/j.jds.2021.04.018. Epub 2021 May 21. J Dent Sci. 2022. PMID: 35028041 Free PMC article.
-
An Outline on the Advancements in Surgical Management of Osteoporosis-Associated Fractures.Cureus. 2024 Jun 26;16(6):e63226. doi: 10.7759/cureus.63226. eCollection 2024 Jun. Cureus. 2024. PMID: 39070522 Free PMC article. Review.
-
Effect of a bioactive glass-based root canal sealer on root fracture resistance ability.J Dent Sci. 2023 Jan;18(1):27-33. doi: 10.1016/j.jds.2022.08.004. Epub 2022 Aug 20. J Dent Sci. 2023. PMID: 36643269 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

