Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy
- PMID: 28620280
- PMCID: PMC5450696
- DOI: 10.3389/fnmol.2017.00174
Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy
Abstract
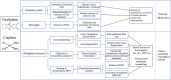
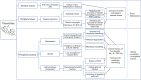
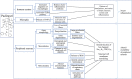
Chemotherapy-induced neuropathy is a common, dose-dependent adverse effect of several antineoplastics. It can lead to detrimental dose reductions and discontinuation of treatment, and severely affects the quality of life of cancer survivors. Clinically, chemotherapy-induced peripheral neuropathy presents as deficits in sensory, motor, and autonomic function which develop in a glove and stocking distribution due to preferential effects on longer axons. The pathophysiological processes are multi-factorial and involve oxidative stress, apoptotic mechanisms, altered calcium homeostasis, axon degeneration and membrane remodeling as well as immune processes and neuroinflammation. This review focusses on the commonly used antineoplastic substances oxaliplatin, cisplatin, vincristine, docetaxel, and paclitaxel which interfere with the cancer cell cycle-leading to cell death and tumor degradation-and cause severe acute and chronic peripheral neuropathies. We discuss drug mechanism of action and pharmacokinetic disposition relevant to the development of peripheral neuropathy, the epidemiology and clinical presentation of chemotherapy-induced neuropathy, emerging insight into genetic susceptibilities as well as current understanding of the pathophysiology and treatment approaches.
Keywords: cancer; chemotherapy; cisplatin; neuropathy; oxaliplatin; paclitaxel; pain; vincristine.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

