Oral Administration of a Seed-based Bivalent Rotavirus Vaccine Containing VP6 and NSP4 Induces Specific Immune Responses in Mice
- PMID: 28620404
- PMCID: PMC5449476
- DOI: 10.3389/fpls.2017.00910
Oral Administration of a Seed-based Bivalent Rotavirus Vaccine Containing VP6 and NSP4 Induces Specific Immune Responses in Mice
Abstract
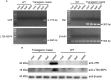
Rotavirus is the leading cause of severe diarrheal disease among newborns. Plant-based rotavirus vaccines have been developed in recent years and have been proven to be effective in animal models. In the present study, we report a bivalent vaccine candidate expressing rotavirus subunits VP6 and NSP4 fused with the adjuvant subunit B of E. coli heat-labile enterotoxin (LTB) in maize seeds. The RT-PCR and Western blot results showed that VP6 and LTB-NSP4 antigens were expressed and accumulated in maize seeds. The expression levels were as high as 0.35 and 0.20% of the total soluble protein for VP6 and LTB-NSP4, respectively. Oral administration of transgenic maize seeds successfully stimulated systemic and mucosal responses, with high titers of serum IgG and mucosal IgA antibodies, even after long-term storage. This study is the first to use maize seeds as efficient generators for the development of a bivalent vaccine against rotavirus.
Keywords: NSP4; VP6; oral vaccine; plant-based vaccine; rotavirus.
Figures




Similar articles
-
Production of Bovine Rotavirus VP6 Subunit Vaccine in a Transgenic Fodder Crop, Egyptian Clover (Berseem, Trifolium alexandrinum) that Elicits Immune Responses in Rabbit.Mol Biotechnol. 2023 Sep;65(9):1432-1443. doi: 10.1007/s12033-022-00648-0. Epub 2023 Jan 13. Mol Biotechnol. 2023. PMID: 36637627
-
Immunization of Mice by Rotavirus NSP4-VP6 Fusion Protein Elicited Stronger Responses Compared to VP6 Alone.Viral Immunol. 2018 Apr;31(3):233-241. doi: 10.1089/vim.2017.0075. Epub 2017 Nov 29. Viral Immunol. 2018. PMID: 29185875
-
Co-administration of rotavirus nanospheres VP6 and NSP4 proteins enhanced the anti-NSP4 humoral responses in immunized mice.Microb Pathog. 2022 Feb;163:105405. doi: 10.1016/j.micpath.2022.105405. Epub 2022 Jan 16. Microb Pathog. 2022. PMID: 35045328
-
VP6: A candidate rotavirus vaccine.J Infect Dis. 2010 Sep 1;202 Suppl:S101-7. doi: 10.1086/653556. J Infect Dis. 2010. PMID: 20684688 Review.
-
Rotavirus VP6 as a potential vaccine candidate.Rev Med Virol. 2019 Mar;29(2):e2027. doi: 10.1002/rmv.2027. Epub 2019 Jan 6. Rev Med Virol. 2019. PMID: 30614135 Review.
Cited by
-
Rotavirus VP6: involvement in immunogenicity, adjuvant activity, and use as a vector for heterologous peptides, drug delivery, and production of nano-biomaterials.Arch Virol. 2022 Apr;167(4):1013-1023. doi: 10.1007/s00705-022-05407-9. Epub 2022 Mar 15. Arch Virol. 2022. PMID: 35292854 Free PMC article. Review.
-
Insights into recent advancements in human and animal rotavirus vaccines: Exploring new frontiers.Virol Sin. 2025 Feb;40(1):1-14. doi: 10.1016/j.virs.2024.12.001. Epub 2024 Dec 11. Virol Sin. 2025. PMID: 39672271 Free PMC article. Review.
-
The Rotavirus Vaccine Landscape, an Update.Pathogens. 2021 Apr 26;10(5):520. doi: 10.3390/pathogens10050520. Pathogens. 2021. PMID: 33925924 Free PMC article. Review.
-
Production of Bovine Rotavirus VP6 Subunit Vaccine in a Transgenic Fodder Crop, Egyptian Clover (Berseem, Trifolium alexandrinum) that Elicits Immune Responses in Rabbit.Mol Biotechnol. 2023 Sep;65(9):1432-1443. doi: 10.1007/s12033-022-00648-0. Epub 2023 Jan 13. Mol Biotechnol. 2023. PMID: 36637627
-
Expression and Purification of Porcine Rotavirus Structural Proteins in Silkworm Larvae as a Vaccine Candidate.Mol Biotechnol. 2023 Mar;65(3):401-409. doi: 10.1007/s12033-022-00548-3. Epub 2022 Aug 13. Mol Biotechnol. 2023. PMID: 35963985 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

