Novel approaches to ultrasonography of the lung and pleural space: where are we now?
- PMID: 28620429
- PMCID: PMC5467658
- DOI: 10.1183/20734735.004717
Novel approaches to ultrasonography of the lung and pleural space: where are we now?
Abstract
This review article is an update of what should be known for practicing basic lung ultrasound in the critically ill (LUCI) and is also of interest for less critical disciplines (e.g. pulmonology). It pinpoints on the necessity of a professional machine (not necessarily a sophisticated one) and probe. It lists the 10 main signs of LUCI and some of the main protocols made possible using LUCI: the BLUE protocol for a respiratory failure, the FALLS protocol for a circulatory failure, the SESAME protocol for a cardiac arrest and the investigation of a ventilated acute respiratory distress syndrome patient, etc. It shows how the field has been fully standardised to avoid confusion.
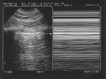
Key points: A simple ultrasonography unit is fully adequate, with minimal filters, and provides a unique probe for integrating the lung into a holistic, whole-body approach to the critically ill.Interstitial syndrome is strictly defined. Its clinical relevance in the critically ill is standardised for defining haemodynamic pulmonary oedema, pneumonia and pulmonary embolism.Pneumothorax is strictly and sequentially defined by the A'-profile (at the anterior wall in a supine or semirecumbent patient, abolished lung siding plus the A-line sign) and then the lung point.The BLUE protocol integrates lung and venous ultrasound findings for expediting the diagnosis of acute respiratory failure, following pathophysiology, allowing prompt diagnosis of pneumonia, haemodynamic pulmonary oedema, exacerbated chronic obstructive pulmonary disease or asthma, pulmonary embolism or pneumothorax, even in clinically challenging presentations.
Educational aims: To understand that the use of lung ultrasound, although long standardised, still needs educational efforts for its best use, a suitable machine, a suitable universal probe and an appropriate culture.To be able to use a terminology that has been fully standardised to avoid any confusion of useless wording.To understand the logic of the BLUE points, three points of interest enabling expedition of a lung ultrasound examination in acute respiratory failure.To be able to cite, in the correct hierarchy, the seven criteria of the B-line, then those of interstitial syndrome.To understand the sequential thinking when making ultrasound diagnosis of pneumothorax.To be able to use the BLUE protocol for building profiles of pneumonia (or acute respiratory distress syndrome) and understand their limitations.To understand that lung ultrasound can be used for the direct analysis of an acute respiratory failure (the BLUE protocol), an acute circulatory failure (the FALLS protocol) and even a cardiac arrest (SESAME protocol), following a pathophysiological approach.To understand that the first sequential target in the SESAME protocol (search first for pneumothorax in cardiac arrest) can also be used in countless more quiet settings of countless disciplines, making lung ultrasound in the critically ill cost-, time- and radiation-saving.To be able to perform a BLUE protocol in challenging patients, understanding how the best lung ultrasound can be obtained from bariatric or agitated, dyspnoeic patients.
Conflict of interest statement
Conflict of interest None declared.
Figures




References
-
- Weinberger SE, Drazen JM. Diagnostic procedures in respiratory diseases In: Harrison’s principles of internal medicine. 16th Edn. New York, McGraw-Hill, 2005; pp. 1505–1508.
-
- Laënnec RTH. Traité de l’Auscultation Médiate, ou Traité du Diagnostic des Maladies des Poumons et du Cœur. Paris, J.A. Brosson & J.S. Chaudé, 1819.
-
- Williams FH. A method for more fully determining the outline of the heart by means of the fluoroscope together with other uses of this instrument in medicine. Boston Med Surg J 1896; 135: 335–337.
-
- Hounsfield GN. Computerized transverse axial scanning. Br J Radiol 1973; 46: 1016–1022. - PubMed
-
- Lichtenstein D. Lung Ultrasound in the Critically Ill: the BLUE Protocol. Heidelberg, Springer-Verlag, 2016.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
