Immunoprofiling of human uterine mast cells identifies three phenotypes and expression of ERβ and glucocorticoid receptor
- PMID: 28620462
- PMCID: PMC5461902
- DOI: 10.12688/f1000research.11432.2
Immunoprofiling of human uterine mast cells identifies three phenotypes and expression of ERβ and glucocorticoid receptor
Abstract
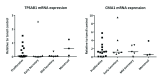
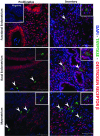
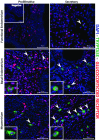
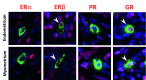
Background: Human mast cells (MCs) are long-lived tissue-resident immune cells characterised by granules containing the proteases chymase and/or tryptase. Their phenotype is modulated by their tissue microenvironment. The human uterus has an outer muscular layer (the myometrium) surrounding the endometrium, both of which play an important role in supporting a pregnancy. The endometrium is a sex steroid target tissue consisting of epithelial cells (luminal, glandular) surrounded by a multicellular stroma, with the latter containing an extensive vascular compartment as well as fluctuating populations of immune cells that play an important role in regulating tissue function. The role of MCs in the human uterus is poorly understood with little known about their regulation or the impact of steroids on their differentiation status. The current study had two aims: 1) To investigate the spatial and temporal location of uterine MCs and determine their phenotype; 2) To determine whether MCs express receptors for steroids implicated in uterine function, including oestrogen (ERα, ERβ), progesterone (PR) and glucocorticoids (GR). Methods: Tissue samples from women (n=46) were used for RNA extraction (n=26) or fixed (n=20) for immunohistochemistry. Results: Messenger RNAs encoded by TPSAB1 (tryptase) and CMA1 (chymase) were detected in endometrial tissue homogenates. Immunohistochemistry revealed the relative abundance of tryptase MCs was myometrium>basal endometrium>functional endometrium. We show for the first time that uterine MCs are predominantly of the classical MC subtypes: (positive, +; negative, -) tryptase+/chymase- and tryptase+/chymase+, but a third subtype was also identified (tryptase-/chymase+). Tryptase+ MCs were of an ERβ+/ERα-/PR-/GR+ phenotype mirroring other uterine immune cell populations, including natural killer cells. Conclusions: Endometrial tissue resident immune MCs have three protease-specific phenotypes. Expression of both ERβ and GR in MCs mirrors that of other immune cells in the endometrium and suggests that MC function may be altered by the local steroid microenvironment.
Keywords: ERb; GR; chymase; oestrogen; steroid receptor; tryptase.
Conflict of interest statement
Competing interests: The authors have no competing interests.
Figures
Similar articles
-
Human mast cells arise from a common circulating progenitor.J Allergy Clin Immunol. 2013 Aug;132(2):463-9.e3. doi: 10.1016/j.jaci.2013.02.011. Epub 2013 Apr 9. J Allergy Clin Immunol. 2013. PMID: 23582567
-
Profiling the expression and function of oestrogen receptor isoform ER46 in human endometrial tissues and uterine natural killer cells.Hum Reprod. 2020 Mar 27;35(3):641-651. doi: 10.1093/humrep/dez306. Hum Reprod. 2020. PMID: 32108901 Free PMC article.
-
Mast cells in diffuse large B-cell lymphoma; their role in fibrosis.Histopathology. 2006 Nov;49(5):498-505. doi: 10.1111/j.1365-2559.2006.02534.x. Histopathology. 2006. PMID: 17064296
-
Significance of mast cells in spermatogenesis, implantation, pregnancy, and abortion: Cross talk and molecular mechanisms.Am J Reprod Immunol. 2020 May;83(5):e13228. doi: 10.1111/aji.13228. Epub 2020 Mar 3. Am J Reprod Immunol. 2020. PMID: 32053232 Review.
-
Mast Cell and Basophil Granule Proteases - In Vivo Targets and Function.Front Immunol. 2022 Jul 5;13:918305. doi: 10.3389/fimmu.2022.918305. eCollection 2022. Front Immunol. 2022. PMID: 35865537 Free PMC article. Review.
Cited by
-
Mast cells selectively produce inflammatory mediators and impact the early response to Chlamydia reproductive tract infection.Front Immunol. 2023 Apr 17;14:1166068. doi: 10.3389/fimmu.2023.1166068. eCollection 2023. Front Immunol. 2023. PMID: 37138882 Free PMC article.
-
The Estrogen-Immune Interface in Endometriosis.Cells. 2025 Jan 6;14(1):58. doi: 10.3390/cells14010058. Cells. 2025. PMID: 39791759 Free PMC article. Review.
-
Female Reproductive Systems: Hormone Dependence and Receptor Expression.Adv Exp Med Biol. 2022;1390:21-39. doi: 10.1007/978-3-031-11836-4_2. Adv Exp Med Biol. 2022. PMID: 36107311
-
Physiomimetic Models of Adenomyosis.Semin Reprod Med. 2020 May;38(2-03):179-196. doi: 10.1055/s-0040-1719084. Epub 2020 Nov 9. Semin Reprod Med. 2020. PMID: 33176387 Free PMC article. Review.
-
Rapid Mast Cell Generation from Gata2 Reporter Pluripotent Stem Cells.Stem Cell Reports. 2018 Oct 9;11(4):1009-1020. doi: 10.1016/j.stemcr.2018.08.007. Epub 2018 Sep 6. Stem Cell Reports. 2018. PMID: 30197119 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

