Infant formula containing galacto-and fructo-oligosaccharides and Bifidobacterium breve M-16V supports adequate growth and tolerance in healthy infants in a randomised, controlled, double-blind, prospective, multicentre study
- PMID: 28620469
- PMCID: PMC5465812
- DOI: 10.1017/jns.2016.35
Infant formula containing galacto-and fructo-oligosaccharides and Bifidobacterium breve M-16V supports adequate growth and tolerance in healthy infants in a randomised, controlled, double-blind, prospective, multicentre study
Abstract
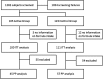
The objective of the present study was to evaluate the growth and tolerance in healthy, term infants consuming a synbiotic formula with daily weight gain as the primary outcome. In a randomised, controlled, double-blind, multicentre, intervention study infants were assigned to an extensively hydrolysed formula containing a specific combination of Bifidobacterium breve M-16V and a prebiotic mixture (short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides in a 9:1 ratio; scGOS/lcFOS; synbiotic group), or the same formula without this synbiotic concept for 13 weeks (control group). Anthropometry, formula intake, tolerance, stool characteristics, blood parameters, faecal microbiota and metabolic faecal profile were assessed. Medically confirmed adverse events were recorded throughout the study. Equivalence in daily weight gain was demonstrated for the intention-to-treat (ITT) population (n 211). In the per-protocol (PP) population (n 102), the 90 % CI of the difference in daily weight gain slightly crossed the lower equivalence margin. During the intervention period, the mean weight-for-age and length-for-age values were close to the median of the WHO growth standards in both groups, indicating adequate growth. The number of adverse events was not different between both groups. No relevant differences were observed in blood parameters indicative for liver and renal function. At 13 weeks, an increased percentage of faecal bifidobacteria (60 v. 48 %) and a reduced percentage of Clostridium lituseburense/C. histolyticum (0·2 v. 2·6 %) were observed in the synbiotic group (n 19) compared with the control group (n 27). In conclusion, this study demonstrates that an extensively hydrolysed formula with B. breve M-16V and the prebiotic mixture scGOS/lcFOS (9:1) supports an adequate infant growth.
Keywords: Bifidobacterium breve M-16V; Hydrolysed formula; ITT, intention-to-treat; Infant growth; PP, per-protocol; RMMM, repeated-measures mixed model; Randomised controlled trials; SCORAD, SCORing Atopic Dermatitis; Synbiotics; scGOS/lcFOS, short-chain galacto-oligosaccharides/long-chain fructo-oligosaccharides.
Figures

References
-
- Field CJ (2005) The immunological components of human milk and their effect on immune development in infants. J Nutr 135, 1–4. - PubMed
-
- Garcia C, Duan RD, Brevaut-Malaty V, et al. (2013) Bioactive compounds in human milk and intestinal health and maturity in preterm newborn: an overview. Cell Mol Biol (Noisy-le-grand) 59, 108–131. - PubMed
-
- Jost T, Lacroix C, Braegger C, et al. (2015) Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr Rev 73, 426–437. - PubMed
-
- Greer FR, Sicherer SH, Burks AW, et al. (2008) Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 121, 183–191. - PubMed
-
- Koletzko S, Niggemann B, Arato A, et al. (2012) Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr 55, 221–229. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

