A prospective, double-blind, randomized, two-period crossover, multicenter study to evaluate tolerability and patient preference between mirabegron and tolterodine in patients with overactive bladder (PREFER study)
- PMID: 28620791
- PMCID: PMC5780540
- DOI: 10.1007/s00192-017-3377-5
A prospective, double-blind, randomized, two-period crossover, multicenter study to evaluate tolerability and patient preference between mirabegron and tolterodine in patients with overactive bladder (PREFER study)
Abstract
Introduction and hypothesis: The objective of this study was to assess the tolerability and treatment preference in patients with overactive bladder (OAB) treated with mirabegron or tolterodine.
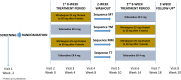
Methods: This was a two-period, 8-week crossover, double-blind, phase IV study (PREFER; NCT02138747) in treatment-naive adults with OAB for 3 months or longer randomized to one of four treatment sequences in a 5:5:1:1 ratio (mirabegron/tolterodine, tolterodine/mirabegron, mirabegron/mirabegron, or tolterodine/tolterodine), separated by a washout period of 2 weeks. The primary endpoint was drug tolerability using the Medication Tolerability scale of the OAB Treatment Satisfaction (OAB-S) questionnaire at end of treatment (EoT). Period-by-treatment interactions were analyzed to determine any effect of drug order. Patient preference, change from baseline in OAB symptoms, and treatment-emergent adverse events (TEAEs) were assessed.
Results: A total of 358 randomized patients completed the OAB-S Medication Tolerability scale questionnaire at one or more visits after the baseline evaluation. The mean (95% CI) OAB-S Medication Tolerability scores were significantly higher (better tolerability) for mirabegron (86.29 [83.50, 89.08]) than for tolterodine (83.40 [80.59, 86.20]; p = 0.004). The period-by-treatment interaction was not significant (p = 0.955). Improvements in OAB-S Medication Tolerability scores at EoT were more evident in women, patients aged ≥65 years, and in patients without baseline incontinence, and were greater with mirabegron than with tolterodine extended release. There were no significant differences in patient preference or improvements in OAB symptoms. Significant differences in favor of mirabegron were observed for anticholinergic TEAEs (20.4% vs. 27.4%; p = 0.042) and specifically for gastrointestinal disorders (14.7% vs. 22.5%; p = 0.015).
Conclusions: Tolerability of mirabegron was significantly higher than that of tolterodine, and patient preference and improvements in OAB symptoms were comparable. Both treatments were well tolerated; however, anticholinergic side effects were higher with tolterodine.
Keywords: Anticholinergic side effects; Crossover study; Mirabegron; OAB-S Medication Tolerability scale; Patient preference; Tolterodine.
Conflict of interest statement
David Staskin has received grants, speaker and consultancy fees from Astellas, speaker and consultancy fees from Allergan, Velicept, StimGuard, NewUro, and Inpellis, and has patents related to surgical devices for stress incontinence with royalties paid.
Sender Herschorn has received grants, speaker and consultancy fees from Astellas, Allergan, and Ipsen, and speaker and consultancy fees from Merus and Pfizer.
Jonathan Fialkov has received investigator fees from Astellas, and is managing director/board chairman of Rational Surgical Solutions, and has received sponsorship fees from Dendreon for Rational Surgical Solutions software application.
Le Mai Tu has received speaker and consultancy fees from Astellas and Pfizer.
Terry Walsh and Carol Schermer are employees of Astellas.
Figures

Similar articles
-
Patient-reported outcomes in patients with overactive bladder treated with mirabegron and tolterodine in a prospective, double-blind, randomized, two-period crossover, multicenter study (PREFER).Health Qual Life Outcomes. 2018 Apr 19;16(1):69. doi: 10.1186/s12955-018-0892-0. Health Qual Life Outcomes. 2018. PMID: 29673355 Free PMC article. Clinical Trial.
-
Results of a randomized, double-blind, parallel-group, placebo- and active-controlled, multicenter study of mirabegron, a β3-adrenoceptor agonist, in patients with overactive bladder in Asia.Neurourol Urodyn. 2015 Sep;34(7):685-92. doi: 10.1002/nau.22645. Epub 2014 Aug 17. Neurourol Urodyn. 2015. PMID: 25130281 Clinical Trial.
-
Patient-reported outcomes with the β3 -adrenoceptor agonist mirabegron in a phase III trial in patients with overactive bladder.Neurourol Urodyn. 2016 Nov;35(8):987-994. doi: 10.1002/nau.22844. Epub 2015 Aug 19. Neurourol Urodyn. 2016. PMID: 26288118 Clinical Trial.
-
Systematic review and meta-analysis on the efficacy and tolerability of mirabegron for the treatment of storage lower urinary tract symptoms/overactive bladder: Comparison with placebo and tolterodine.Int J Urol. 2018 Mar;25(3):196-205. doi: 10.1111/iju.13498. Epub 2017 Dec 3. Int J Urol. 2018. PMID: 29205506
-
Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability.Neurourol Urodyn. 2014 Jan;33(1):17-30. doi: 10.1002/nau.22505. Epub 2013 Oct 11. Neurourol Urodyn. 2014. PMID: 24127366 Review.
Cited by
-
Mirabegron in the Management of Overactive Bladder Syndrome.Int J Womens Health. 2022 Sep 16;14:1337-1350. doi: 10.2147/IJWH.S372597. eCollection 2022. Int J Womens Health. 2022. PMID: 36147890 Free PMC article. Review.
-
Patient-reported outcomes in patients with overactive bladder treated with mirabegron and tolterodine in a prospective, double-blind, randomized, two-period crossover, multicenter study (PREFER).Health Qual Life Outcomes. 2018 Apr 19;16(1):69. doi: 10.1186/s12955-018-0892-0. Health Qual Life Outcomes. 2018. PMID: 29673355 Free PMC article. Clinical Trial.
-
Mirabegron 50 mg once daily, long-term treatment maximizes benefit in middle-aged and older people with overactive bladder syndrome: a systematic review and meta-analysis of nine phase II/III, randomized, double-blind, parallel-design, placebo-controlled, multicenter, and multinational trials.Front Surg. 2024 Aug 26;11:1372175. doi: 10.3389/fsurg.2024.1372175. eCollection 2024. Front Surg. 2024. PMID: 39252844 Free PMC article.
-
Highly Enantioselective Synthesis of 3,3-Diarylpropyl Amines and 4-Aryl Tetrahydroquinolines via Ir-Catalyzed Asymmetric Hydrogenation.Org Lett. 2024 Dec 20;26(50):10903-10909. doi: 10.1021/acs.orglett.4c04076. Epub 2024 Dec 5. Org Lett. 2024. PMID: 39636659 Free PMC article.
-
Mirabegron is alternative to antimuscarinic agents for overactive bladder without higher risk in hypertension: a systematic review and meta-analysis.World J Urol. 2018 Aug;36(8):1285-1297. doi: 10.1007/s00345-018-2268-9. Epub 2018 Mar 19. World J Urol. 2018. PMID: 29556972
References
-
- Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological association (IUGA)/International continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26. doi: 10.1007/s00192-009-0976-9. - DOI - PubMed
-
- Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int. 2008;101(11):1388–1395. doi: 10.1111/j.1464-410X.2008.07601.x. - DOI - PubMed
-
- Maman K, Aballea S, Nazir J, Desroziers K, Neine ME, Siddiqui E, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol. 2014;65(4):755–765. doi: 10.1016/j.eururo.2013.11.010. - DOI - PubMed
-
- Khullar V, Amarenco G, Angulo JC, Cambronero J, Hoye K, Milsom I, et al. Efficacy and tolerability of mirabegron, a beta(3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European-Australian phase 3 trial. Eur Urol. 2013;63(2):283–295. doi: 10.1016/j.eururo.2012.10.016. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

