Morphology and evolution of the oral shield in marsupial neonates including the newborn monito del monte (Dromiciops gliroides, Marsupialia Microbiotheria) pouch young
- PMID: 28620997
- PMCID: PMC5472534
- DOI: 10.1111/joa.12621
Morphology and evolution of the oral shield in marsupial neonates including the newborn monito del monte (Dromiciops gliroides, Marsupialia Microbiotheria) pouch young
Abstract
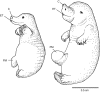
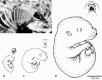
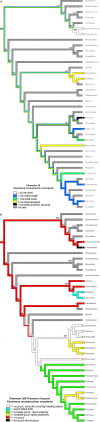
Newborn marsupials can be arranged into three grades of developmental complexity based on their external form, as well as based on their organ systems and their cytology. The dasyurids are considered the least developed marsupials at birth, while didelphids and peramelids are intermediate, and macropods are the most developed. Currently there is still little information on caenolestid and microbiotherid development at birth. Developmental stages can be graded as G1, G2 and G3, with G1 being the least developed at birth, and G3 the most developed. Marsupials are also characterized by having an extremely developed craniofacial region at birth compared with placentals. However, the facial region is also observed to vary in development between different marsupial groups at birth. The oral shield is a morphological structure observed in the oral region of the head during late embryological development, which will diminish shortly after birth. Morphological variation of the oral shield is observed and can be arranged by developmental complexity from greatly developed, reduced to vestigial. In its most developed state, the lips are fused, forming together with the rhinarium, a flattened ring around the buccal opening. In this study, we examine the external oral shield morphology in different species of newborn marsupials (dasyurids, peramelids, macropods and didelphids), including the newborn monito del monte young (Dromiciops gliroides - the sole survivor of the order Microbiotheria). The adaptive value of the oral shield structure is reviewed, and we discuss if this structure may be influenced by developmental stage of newborn, pouch cover, species relatedness, or other reproductive features. We observe that the oral shield structure is present in most species of Marsupialia and appears to be exclusively present in this infraclass. It has never been described in Monotremata or Eutherians. It is present in unrelated taxa (e.g. didelphids, dasyurids and microbiotherids). We observe that a well-developed oral shield may be related to ultra altricial development at birth, large litter size (more than two), and is present in most species that lack a pouch in reproductive adult females or have a less prominent or less developed pouch with some exceptions. We try to explore the evolution of the oral shield structure using existing databases and our own observations to reconstruct likely ancestral character states that can then be used to estimate the evolutionary origin of this structure and if it was present in early mammals. We find that a simple to develop oral shield structure (type 2-3) may have been present in marsupial ancestors as well as in early therians, even though this structure is not present in the extant monotremes. This in turn may suggest that early marsupials may have had a very simple pouch or lacked a pouch as seen in some living marsupials, such as some dasyurids, didelphids and caenolestids. The study's results also suggest that different morphological stages of the oral shield and hindlimb development may be influenced by species size and reproductive strategy, and possibly by yet unknown species-specific adaptations.
Keywords: Dromiciops gliroides; marsupial; monotreme; newborn; oral shield.
© 2017 Anatomical Society.
Figures



References
-
- Amico GC, Rodríguez‐Cabal MA and Aizen MA (2009) The potential key seed‐dispersing role of the arboreal marsupial Dromiciops gliroides . Acta Oecol 35, 8–13.
-
- Amrine‐Madsen H, Scally M, Westerman M, et al. (2003) Nuclear gene sequences provide evidence for the monophyly of australidelphian marsupials. Mol Phylogenet Evol 28, 186–196. - PubMed
-
- Aplin KP, Helgen KM, Lunde DP (2010) A review of Peroryctes broadbenti, the giant bandicoot of Papua New Guinea. Am Mus Novit 3696, 1–41.
-
- Ashwell KWS ed. (2010) The Neurobiology of Australian marsupials: Brain Evolution in the Other Mammalian Radiation. Cambridge, UK: Cambridge University Press.
-
- Ashwell KWS (2013) Neurobiology of Monotremes: Brain Evolution in Our Distant Mammalian Cousins. Collingwood, Australia: CSIRO Publishing.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

