Depletion of FoxP3+ Tregs improves control of larval Echinococcus multilocularis infection by promoting co-stimulation and Th1/17 immunity
- PMID: 28621034
- PMCID: PMC5691311
- DOI: 10.1002/iid3.181
Depletion of FoxP3+ Tregs improves control of larval Echinococcus multilocularis infection by promoting co-stimulation and Th1/17 immunity
Abstract
Introduction: The growth potential of the tumor-like Echinococcus multilocularis metacestode (causing alveolar echinococcosis, AE) is directly linked to the nature/function of the periparasitic host immune-mediated processes. Previous studies had shown that regulatory T cells (Tregs) become gradually up-regulated in the course of both chronic human and murine AE. Thus we now tackled the role of FoxP3+ Tregs and FoxP3+ -Treg-regulated immune response in contributing to the control of this helminthic infection.
Methods: The infection outcome in E. multilocularis-infected DEREG mice was measured upon determining parasite load (wet weight of parasitic metacestode tissue). Flow cytometry and qRT-PCR were used to assess Treg, Th17-, Th1-, Th2-type immune responses and antigen presenting cell activation.
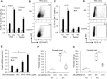
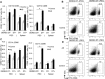
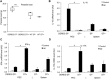
Results: We showed that E. multilocularis-infected DEREG-mice treated with DT (as compared to infected control DEREG-mice without DT application) exhibited a significantly lower parasite load, associated with a persisting capacity of co-stimulation, and an increased Th1/Th17-polarization.
Conclusions: FoxP3+ Tregs appear as one of the key players in immune regulatory processes favoring (i) metacestode survival by inhibiting the maturation potential of co-stimulatory activity and (ii) T cell exhaustion (suppressing Th1/Th17-type immune responses). We showed as well that prospectively, targeting FoxP3+ Tregs could be an option to develop an immunotherapy against AE.
Keywords: CD4+ CD25+ Treg; Echinococcus multilocularis; Foxp3; Th1/Th17 immunity; co-stimulation.
© 2017 The Authors. Immunity, Inflammation and Disease Published by John Wiley & Sons Ltd.
Figures



References
-
- Vuitton, D. A. 2003. The ambiguous role of immunity in echinococcosis: protection of the host or of the parasite? Acta. Trop. 85:119–132. - PubMed
-
- Vuitton, D. A. , Zhang S. L., Yang Y., Godot V., Beurton I., Mantion G., and Bresson‐Hadni S.. 2006. Survival strategy of Echinococcus multilocularis in the human host. Parasitol. Int. 55(Suppl):S51–S55. - PubMed
-
- Wang, J. , Lin R., Zhang W., Li L., Gottstein B., Blagosklonov O., Lu G., Zhang C., Lu X., Vuitton D. A., et al. 2014. Transcriptional profiles of cytokine/chemokine factors of immune cell‐homing to the parasitic lesions: a comprehensive one‐year course study in the liver of E. multilocularis‐infected mice. PLoS ONE 9:e91638. - PMC - PubMed
-
- Tuxun, T. , Wang J. H., Lin R. Y., Shan J. Y., Tai Q. W., Li T., Zhang J. H., Zhao J. M., and Wen H.. 2012. Th17/Treg imbalance in patients with liver cystic echinococcosis. Parasite. Immunol. 34:520–527. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

