Changes to International Nonproprietary Names for antibody therapeutics 2017 and beyond: of mice, men and more
- PMID: 28621572
- PMCID: PMC5590622
- DOI: 10.1080/19420862.2017.1341029
Changes to International Nonproprietary Names for antibody therapeutics 2017 and beyond: of mice, men and more
Abstract
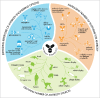
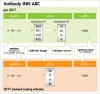
Active pharmaceutical substances require an International Nonproprietary Name (INN) assigned by the World Health Organization (WHO) to obtain market authorization as a medicinal product. INNs are selected to represent a unique, generic name for a drug enabling unambiguous identification by stakeholders worldwide. INNs may be requested after initiating clinical development of an investigational drug. Pharmaceutical classes are indicated by a common stem or suffix. Currently, INNs for monoclonal antibody-based drugs are recognized by the suffix, -mab, preceded by a source infix such as -xi- (chimeric), -zu- (humanized) or -u- (human) designating the species from which the antibody was derived. However, many technological advances have made it increasingly difficult to accurately capture an antibody's source in its name. In 2014, the WHO and the United States Adopted Names (USAN) Council approached this challenge by implementing changes to antibody source infix definitions. Unfortunately, gaps and ambiguities in the definitions and procedures resulted in inconsistent source category assignments and widespread confusion. The Antibody Society, extensively supported by academic and industry scientists, voiced concerns leading to constructive dialog during scheduled consultations with WHO and USAN Council representatives. In June 2017, the WHO announced that use of the source infix will be discontinued for new antibody INNs effective immediately. We fully support this change as it better aligns antibody INNs with current and foreseeable future innovations in antibody therapeutics. Here we review the changes implemented. Additionally, we analyzed antibody INNs recently assigned under the previous 2014 definitions and provide recommendations for further alignment.
Keywords: Chimeric; INN; International Nonproprietary Name; USAN; World Health Organization; drug development; humanization; immunotherapy; monoclonal antibody; therapeutic antibody.
Figures

References
-
- Burns R. To a mouse, on turning her up in her nest with the plough. 1785. The Complete Works of Robert Burns. http://www.robertburns.org/works/75.shtml
-
- Jones TD, Carter PJ, Plückthun A, Vasquez M, Holgate RG, Hotzel I, Popplewell AG, Parren PW, Enzelberger M, Rademaker HJ, et al.. The INNs and outs of antibody nonproprietary names. MAbs 2016; 8:1-9; PMID:26716992; https://doi.org/10.1080/19420862.2015.1114320 - DOI - PMC - PubMed
-
- World Health Organization International non-proprietary names for pharmaceutical substances (INNs). RECOMMENDED international nonproprietary names: List 77. WHO Drug Inform 2017; 31(1): 1-150. http://www.who.int/medicines/publications/druginformation/issues/77_INN_...
-
- World Health Organization 62nd Consultation on International Nonproprietary Names for Pharmaceutical Substances. Geneva, 12–15 April 2016. INN Working Doc. 16.395. July 2016. http://www.who.int/medicines/services/inn/62nd_Executive_Summary.pdf?ua=1
-
- Gillies SD, Lo KM, Wesolowski J. High-level expression of chimeric antibodies using adapted cDNA variable region cassettes. J Immunol Methods 1989; 125:191-202; PMID:2514231; https://doi.org/10.1016/0022-1759(89)90093-8 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
