Short-duration physical activity prevents the development of activity-induced hyperalgesia through opioid and serotoninergic mechanisms
- PMID: 28621702
- PMCID: PMC5561491
- DOI: 10.1097/j.pain.0000000000000967
Short-duration physical activity prevents the development of activity-induced hyperalgesia through opioid and serotoninergic mechanisms
Abstract
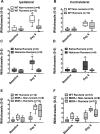
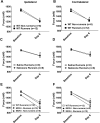
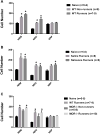
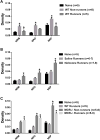
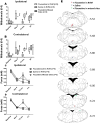
Regular physical activity prevents the development of chronic muscle pain through the modulation of central mechanisms that involve rostral ventromedial medulla (RVM). We tested if pharmacological blockade or genetic deletion of mu-opioid receptors in physically active mice modulates excitatory and inhibitory systems in the RVM in an activity-induced hyperalgesia model. We examined response frequency to mechanical stimulation of the paw, muscle withdrawal thresholds, and expression of phosphorylation of the NR1 subunit of the N-methyl-D-aspartate receptor (p-NR1) and serotonin transporter (SERT) in the RVM. Mice that had performed 5 days of voluntary wheel running prior to the induction of the model were compared with sedentary mice. Sedentary mice showed significant increases in mechanical paw withdrawal frequency and a reduction in muscle withdrawal threshold; wheel running prevented the increase in paw withdrawal frequency. Naloxone-treated and MOR mice had increases in withdrawal frequency that were significantly greater than that in physically active control mice and similar to sedentary mice. Immunohistochemistry in the RVM showed increases in p-NR1 and SERT expression in sedentary mice 24 hours after the induction of the model. Wheel running prevented the increase in SERT, but not p-NR1. Physically active, naloxone-treated, and MOR mice showed significant increases in SERT immunoreactivity when compared with wild-type physically active control mice. Blockade of SERT in the RVM in sedentary mice reversed the activity-induced hyperalgesia of the paw and muscle. These results suggest that analgesia induced by 5 days of wheel running is mediated by mu-opioid receptors through the modulation of SERT, but not p-NR1, in RVM.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Arvidsson U, Cullheim S, Ulfhake B, Ramírez V, Dagerlind Å, Luppi PH, Kitahama K, Jouvet M, Terenius L, Åman K. Distribution of enkephalin and its relation to serotonin in cat and monkey spinal cord and brain stem. Synapse. 1992;11(2):85–104. - PubMed
-
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7(1):309–338. - PubMed
-
- Bement MKH, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil. 2005;86(9):1736–1740. - PubMed
-
- Bidonde J, Jean Busch A, Bath B, Milosavljevic S. Exercise for adults with fibromyalgia: an umbrella systematic review with synthesis of best evidence. Current rheumatology reviews. 2014;10(1):45–79. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

