A Recyclable Cu-MOF-74 Catalyst for the Ligand-Free O-Arylation Reaction of 4-Nitrobenzaldehyde and Phenol
- PMID: 28621710
- PMCID: PMC5485796
- DOI: 10.3390/nano7060149
A Recyclable Cu-MOF-74 Catalyst for the Ligand-Free O-Arylation Reaction of 4-Nitrobenzaldehyde and Phenol
Abstract
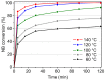
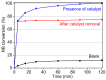
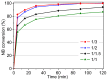
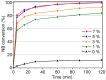
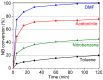
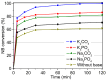
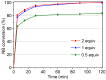
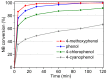
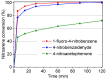
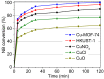
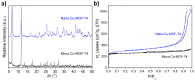
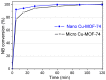
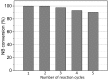
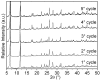
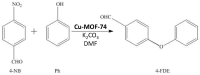
The activity and recyclability of Cu-MOF-74 as a catalyst was studied for the ligand-free C-O cross-coupling reaction of 4-nitrobenzaldehyde (NB) with phenol (Ph) to form 4-formyldiphenyl ether (FDE). Cu-MOF-74 is characterized by having unsaturated copper sites in a highly porous metal-organic framework. The influence of solvent, reaction temperature, NB/Ph ratio, catalyst concentration, and basic agent (type and concentration) were evaluated. High conversions were achieved at 120 °C, 5 mol % of catalyst, NB/Ph ratio of 1:2, DMF as solvent, and 1 equivalent of K₂CO₃ base. The activity of Cu-MOF-74 material was higher than other ligand-free copper catalytic systems tested in this study. This catalyst was easily separated and reused in five successive runs, achieving a remarkable performance without significant porous framework degradation. The leaching of copper species in the reaction medium was negligible. The O-arylation between NB and Ph took place only in the presence of Cu-MOF-74 material, being negligible without the solid catalyst. The catalytic advantages of using nanostructured Cu-MOF-74 catalyst were also proven.
Keywords: 4-formyldiphenyl ether; 4-nitrobenzaldehyde; MOF; O-arylation reaction; catalyst; ligand-free; phenol; recyclable Cu-MOF-74.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lindley J. Copper assisted nucleophilic substitution of aryl halogen. Tetrahedron. 1984;40:1433–1456. doi: 10.1016/S0040-4020(01)91791-0. - DOI
-
- Choudhary V.R., Dumbre D.K., Yadav P.N., Bhargava S.K. Thermally decomposed Cu-Fe-hydrotalcite: A novel highly active catalyst for o-arylation of naphthol and phenols by aryl halides. Catal. Commun. 2012;29:132–136. doi: 10.1016/j.catcom.2012.09.024. - DOI
-
- Benyahya S., Monnier F., Taillefer M., Man M.W.C., Bied C., Ouazzani F. Efficient and Versatile Sol–Gel Immobilized Copper Catalyst for Ullmann Arylation of Phenols. Adv. Synth. Catal. 2008;350:2205–2208. doi: 10.1002/adsc.200800360. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

