Anti-Inflammatory and Anti-Oxidant Potential of the Root Extract and Constituents of Doronicum austriacum
- PMID: 28621735
- PMCID: PMC6152664
- DOI: 10.3390/molecules22061003
Anti-Inflammatory and Anti-Oxidant Potential of the Root Extract and Constituents of Doronicum austriacum
Abstract
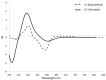
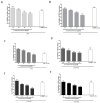
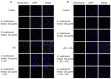
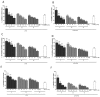
Doronicum austriacum Jacq., Asteraceae, is a plant which is used in traditional alpine medicine. Historical sources describe the medical use of the root, but up until now only a few studies evaluated its pharmacological properties. The evaluation of the dichloromethane extract, and its major compounds for their anti-inflammatory and anti-oxidant potential was performed in macrophages J774A.1 and C6 astrocytes. Nitric oxide (NO) and reactive oxygen species (ROS) release, as well as nitrotyrosine formation, were evaluated. Moreover, in order to evaluate the potential anti-proliferative activity, under the same experimental conditions, 3-(4,5-dimethyltiazol-2yl)-2,5-phenyl-2H-tetrazolium bromide (MTT) assay was also performed. Our results indicate that Doronicum austriacum has a significant effect in inhibiting both pro-inflammatory and pro-oxidative mediators. All isolated compounds were able to significantly inhibit NO and ROS release both in macrophage and in astrocytes cells, even if the effect was more pronounced in macrophage. In particular, among the tested compounds, 6,12-dihydroxy-(-)-2S-tremetone exerted stronger activity. Both extract and single compounds did not affect cellular viability. This study provides evidence for the pharmacological anti-inflammatory and anti-oxidant potential of Doronicum austriacum extract. These effects could be due to the activity of its major constituents and subsequent identification of benzofurans as a promising compound class to combat inflammation and related diseases.
Keywords: Doronicum austriacum; NO; ROS; astrocytes; benzofurans; inflammation; macrophages; nitrotyrosine; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
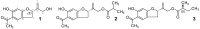
References
-
- Pike J., Chandra R.K. Effect of vitamin and trace element supplementation on immune indices in healthy elderly. Int. J. Vitam. Nutr. Res. 1995;65:117–121. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

